Abstract
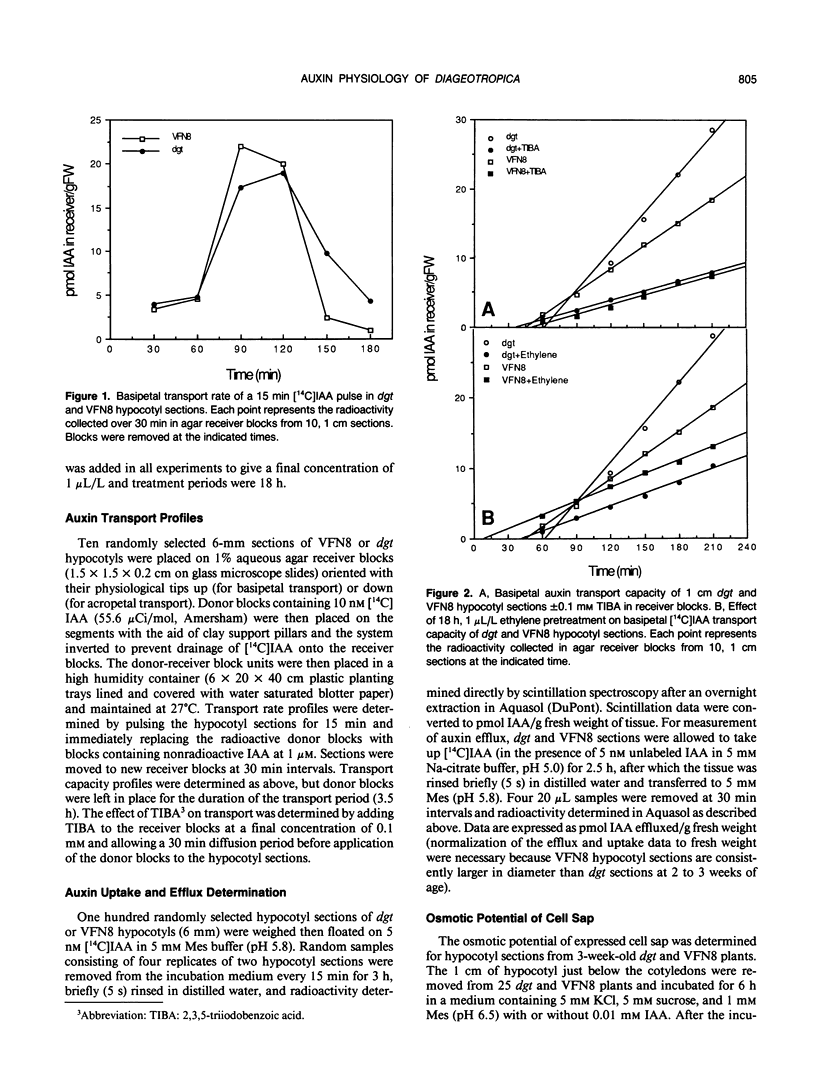
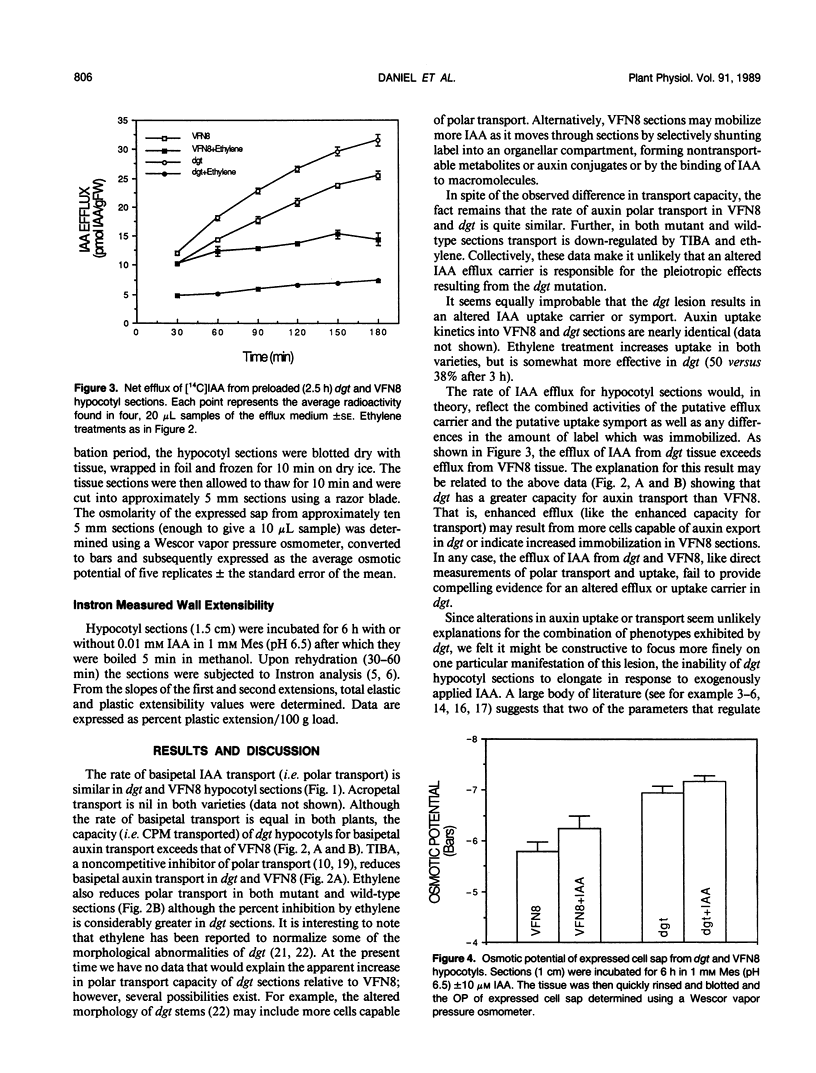
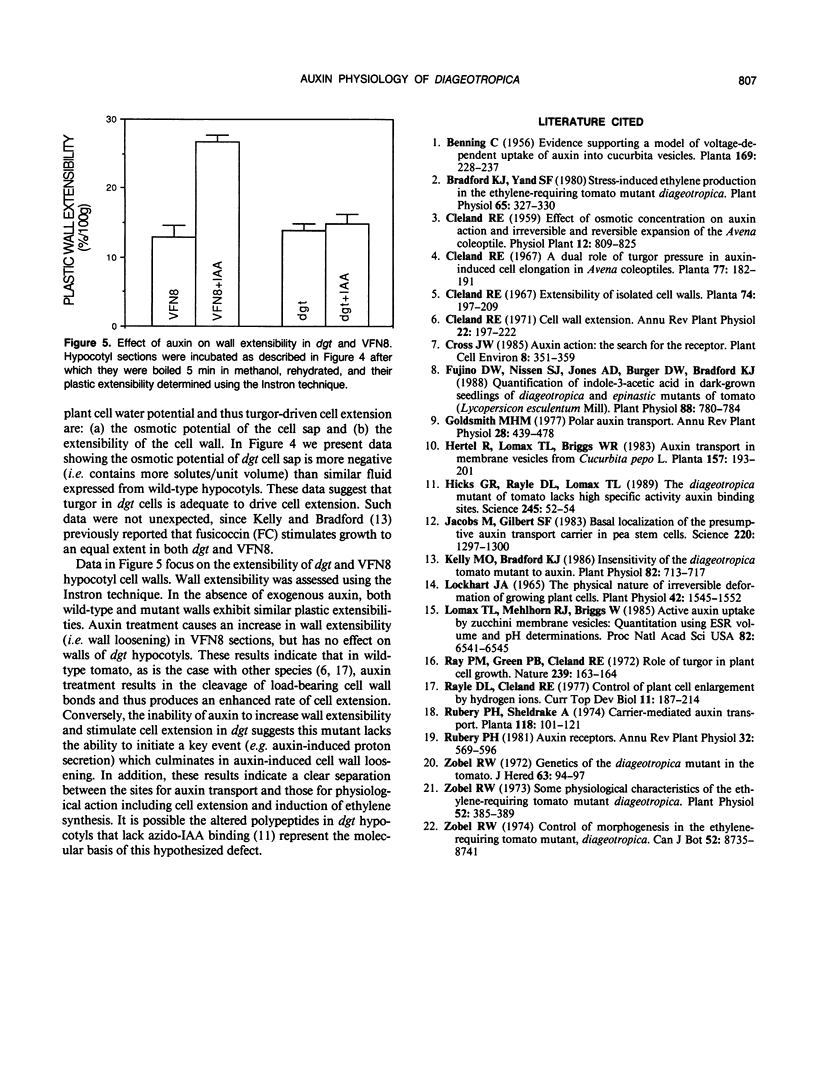
The tomato (Lycopersicon esculentum, Mill.) mutant diageotropica (dgt) exhibits biochemical, physiological, and morphological abnormalities that suggest the mutation may have affected a primary site of auxin perception or action. We have compared two aspects of the auxin physiology of dgt and wild-type (VFN8) seedlings: auxin transport and cellular growth parameters. The rates of basipetal indole-3-acetic acid (IAA) polar transport are identical in hypocotyl sections of the two genotypes, but dgt sections have a slightly greater capacity for IAA transport. 2,3,5-Triiodobenzoic acid and ethylene reduce transport in both mutant and wild-type sections. The kinetics of auxin uptake into VFN8 and dgt sections are nearly identical. These results make it unlikely that an altered IAA efflux carrier or IAA uptake symport are responsible for the pleiotropic effects resulting from the dgt mutation. The lack of auxin-induced cell elongation in dgt plants is not due to insufficient turgor, as the osmotic potential of dgt cell sap is less (more negative) than that of VFN8. An auxin-induced increase in wall extensibility, as measured by the Instron technique, only occurs in the VFN8 plants. These data suggest dgt hypocotyls suffer a defect in the sequence of events culminating in auxin-induced cell wall loosening.
Full text
PDF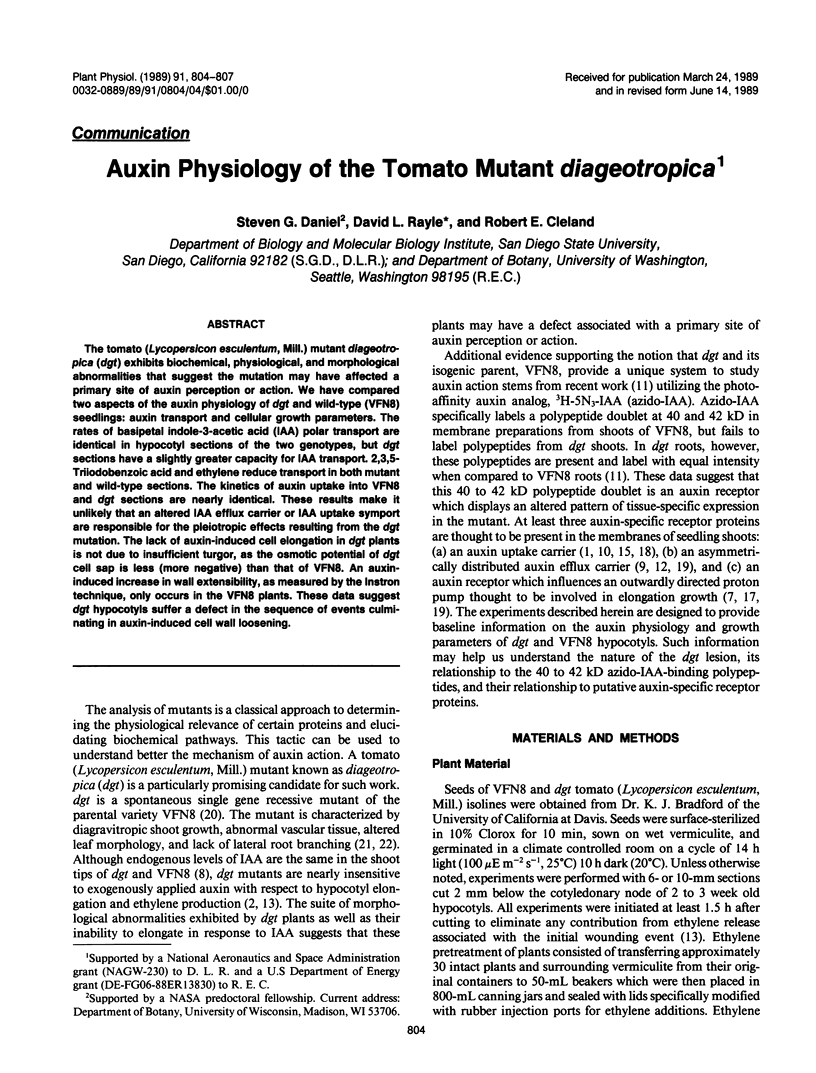
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford K. J., Yang S. F. Stress-induced Ethylene Production in the Ethylene-requiring Tomato Mutant Diageotropica. Plant Physiol. 1980 Feb;65(2):327–330. doi: 10.1104/pp.65.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino D. W., Nissen S. J., Jones A. D., Burger D. W., Bradford K. J. Quantification of Indole-3-Acetic Acid in Dark-Grown Seedlings of the Diageotropica and Epinastic Mutants of Tomato (Lycopersicon esculentum Mill.). Plant Physiol. 1988 Nov;88(3):780–784. doi: 10.1104/pp.88.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks G. R., Rayle D. L., Lomax T. L. The diageotropica mutant of tomato lacks high specific activity auxin binding sites. Science. 1989 Jul 7;245:52–54. doi: 10.1126/science.245.4913.52. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Gilbert S. F. Basal localization of the presumptive auxin transport carrier in pea stem cells. Science. 1983 Jun 17;220(4603):1297–1300. doi: 10.1126/science.220.4603.1297. [DOI] [PubMed] [Google Scholar]
- Kelly M. O., Bradford K. J. Insensitivity of the diageotropica tomato mutant to auxin. Plant Physiol. 1986 Nov;82(3):713–717. doi: 10.1104/pp.82.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart J. A. Physical nature of irreversible deformation of plant cells. Plant Physiol. 1967 Nov;42(11):1545–1552. doi: 10.1104/pp.42.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax T. L., Mehlhorn R. J., Briggs W. R. Active auxin uptake by zucchini membrane vesicles: quantitation using ESR volume and delta pH determinations. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6541–6545. doi: 10.1073/pnas.82.19.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol. 1977;11:187–214. doi: 10.1016/s0070-2153(08)60746-2. [DOI] [PubMed] [Google Scholar]
- Zobel R. W. Some Physiological Characteristics of the Ethylene-requiring Tomato Mutant Diageotropica. Plant Physiol. 1973 Oct;52(4):385–389. doi: 10.1104/pp.52.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]


