Abstract
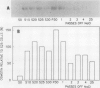
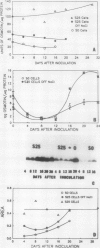
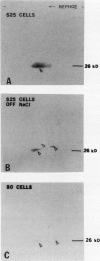
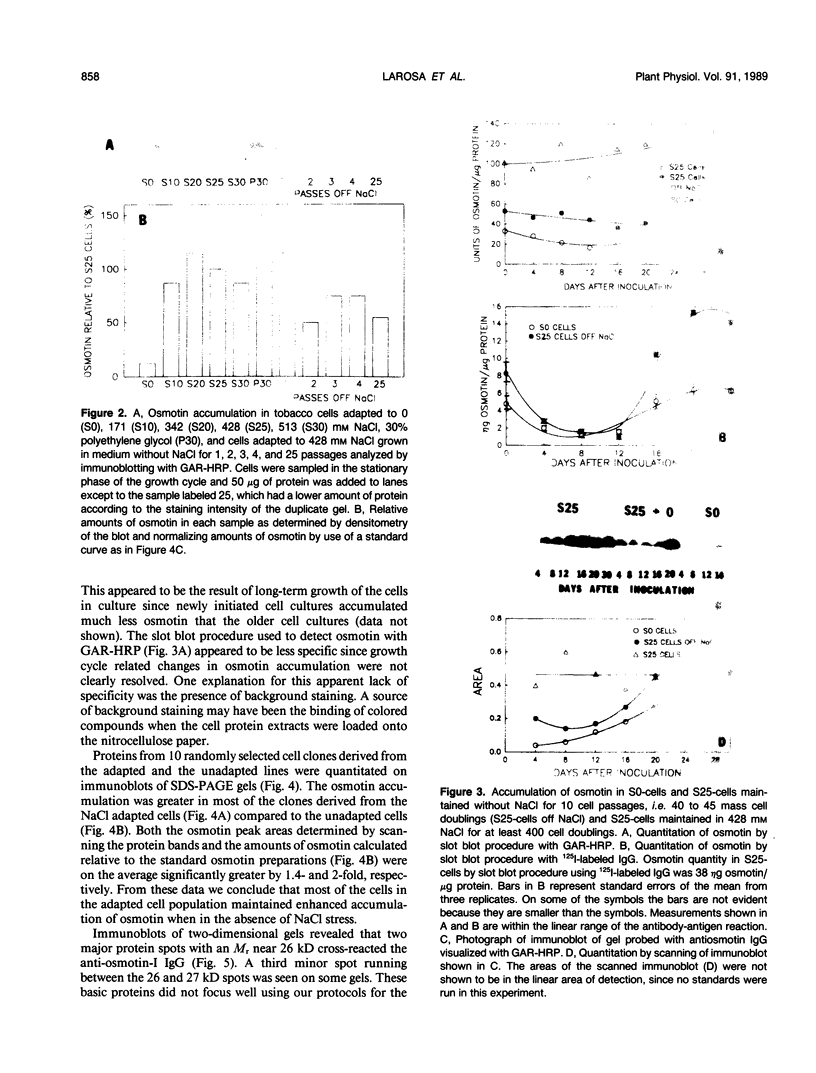
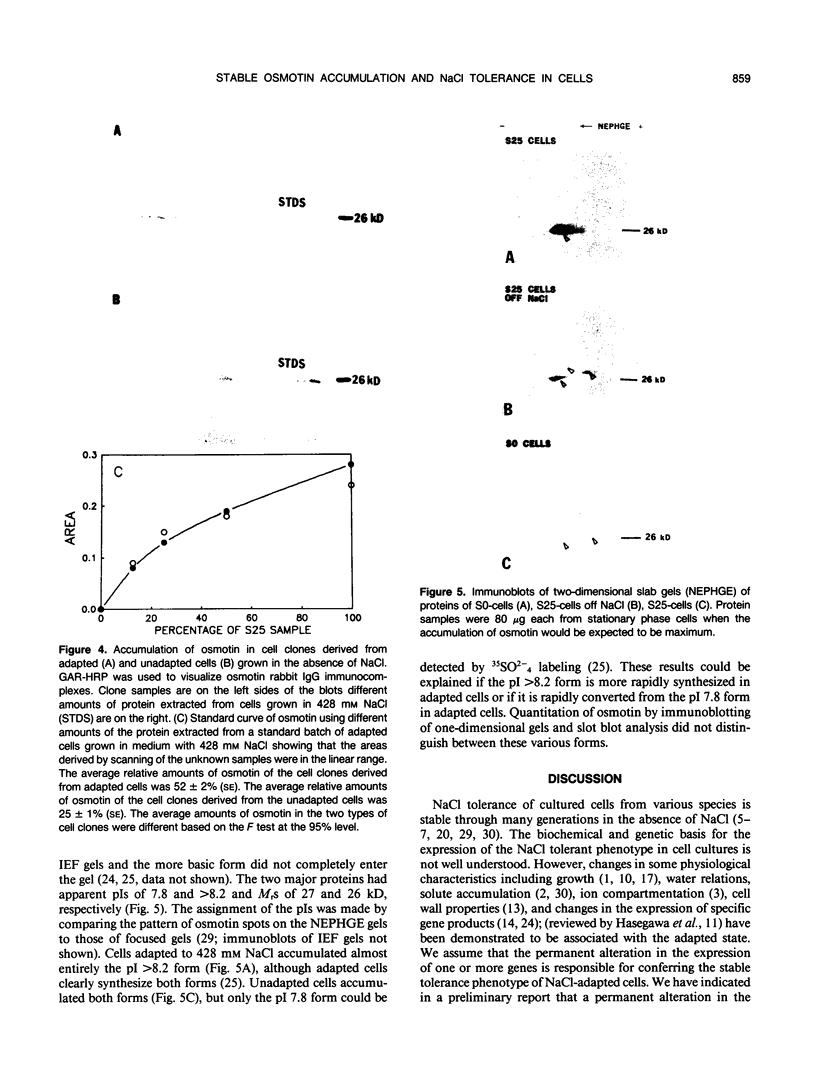
Osmotin is a major protein which accumulates in tobacco cells (Nicotiana tabacum L. var Wisconsin 38) adapted to low water potentials. Quantitation of osmotin levels by immunoblots indicated that cells adapted to 428 millimolar NaCl contained 4 to 30 times the level of osmotin found in unadapted cells, depending on the stage of growth. Unadapted cells accumulated low levels of osmotin with apparent isoelectric points, (pl) of 7.8 and >8.2. Upon transfer of NaCl-adapted cells to medium without NaCl and subsequent growth for many cell generations, the amount of osmotin declined gradually to a level intermediate between that found in adapted and unadapted cells. NaCl-adapted cells grown in the absence of NaCl accumulated both pl forms; however, the form accumulated by cells adapted to NaCl (pl > 8.2) was most abundant. Adapted cells grown in the absence of NaCl exhibited absolute growth rates and NaCl tolerance levels which were intermediate to those of NaCl-adapted and unadapted cells. The association between osmotin accumulation and stable NaCl tolerance indicates that cells with a stable genetic change affecting the accumulation of osmotin are selected during prolonged exposure to high levels of NaCl. This stable alteration in gene expression probably affects salt tolerance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binzel M. L., Hasegawa P. M., Handa A. K., Bressan R. A. Adaptation of Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):118–125. doi: 10.1104/pp.79.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M. L., Hasegawa P. M., Rhodes D., Handa S., Handa A. K., Bressan R. A. Solute Accumulation in Tobacco Cells Adapted to NaCl. Plant Physiol. 1987 Aug;84(4):1408–1415. doi: 10.1104/pp.84.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M. L., Hess F. D., Bressan R. A., Hasegawa P. M. Intracellular compartmentation of ions in salt adapted tobacco cells. Plant Physiol. 1988 Feb;86(2):607–614. doi: 10.1104/pp.86.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Gulick P., Dvorák J. Gene induction and repression by salt treatment in roots of the salinity-sensitive Chinese Spring wheat and the salinity-tolerant Chinese Spring x Elytrigia elongata amphiploid. Proc Natl Acad Sci U S A. 1987 Jan;84(1):99–103. doi: 10.1073/pnas.84.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa A. K., Bressan R. A., Handa S., Hasegawa P. M. Clonal variation for tolerance to polyethylene glycol-induced water stress in cultured tomato cells. Plant Physiol. 1983 Jul;72(3):645–653. doi: 10.1104/pp.72.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. The effects of salt on the pattern of protein synthesis in barley roots. Plant Physiol. 1987 Mar;83(3):517–524. doi: 10.1104/pp.83.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraki N. M., Singh N., Bressan R. A., Carpita N. C. Cell Walls of Tobacco Cells and Changes in Composition Associated with Reduced Growth upon Adaptation to Water and Saline Stress. Plant Physiol. 1989 Sep;91(1):48–53. doi: 10.1104/pp.91.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Abscisic Acid accelerates adaptation of cultured tobacco cells to salt. Plant Physiol. 1985 Sep;79(1):138–142. doi: 10.1104/pp.79.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larosa P. C., Hasegawa P. M., Rhodes D., Clithero J. M., Watad A. E., Bressan R. A. Abscisic Acid Stimulated Osmotic Adjustment and Its Involvement in Adaptation of Tobacco Cells to NaCl. Plant Physiol. 1987 Sep;85(1):174–181. doi: 10.1104/pp.85.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Ramagopal S. Differential mRNA transcription during salinity stress in barley. Proc Natl Acad Sci U S A. 1987 Jan;84(1):94–98. doi: 10.1073/pnas.84.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Bracker C. A., Hasegawa P. M., Handa A. K., Buckel S., Hermodson M. A., Pfankoch E., Regnier F. E., Bressan R. A. Characterization of osmotin : a thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987 Oct;85(2):529–536. doi: 10.1104/pp.85.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Handa A. K., Hasegawa P. M., Bressan R. A. Proteins Associated with Adaptation of Cultured Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):126–137. doi: 10.1104/pp.79.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Hormonal regulation of protein synthesis associated with salt tolerance in plant cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):739–743. doi: 10.1073/pnas.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Nelson D. E., Kuhn D., Hasegawa P. M., Bressan R. A. Molecular Cloning of Osmotin and Regulation of Its Expression by ABA and Adaptation to Low Water Potential. Plant Physiol. 1989 Jul;90(3):1096–1101. doi: 10.1104/pp.90.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watad A. E., Reinhold L., Lerner H. R. Comparison between a Stable NaCl-Selected Nicotiana Cell Line and the Wild Type : K, Na, and Proline Pools as a Function of Salinity. Plant Physiol. 1983 Nov;73(3):624–629. doi: 10.1104/pp.73.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]