Abstract
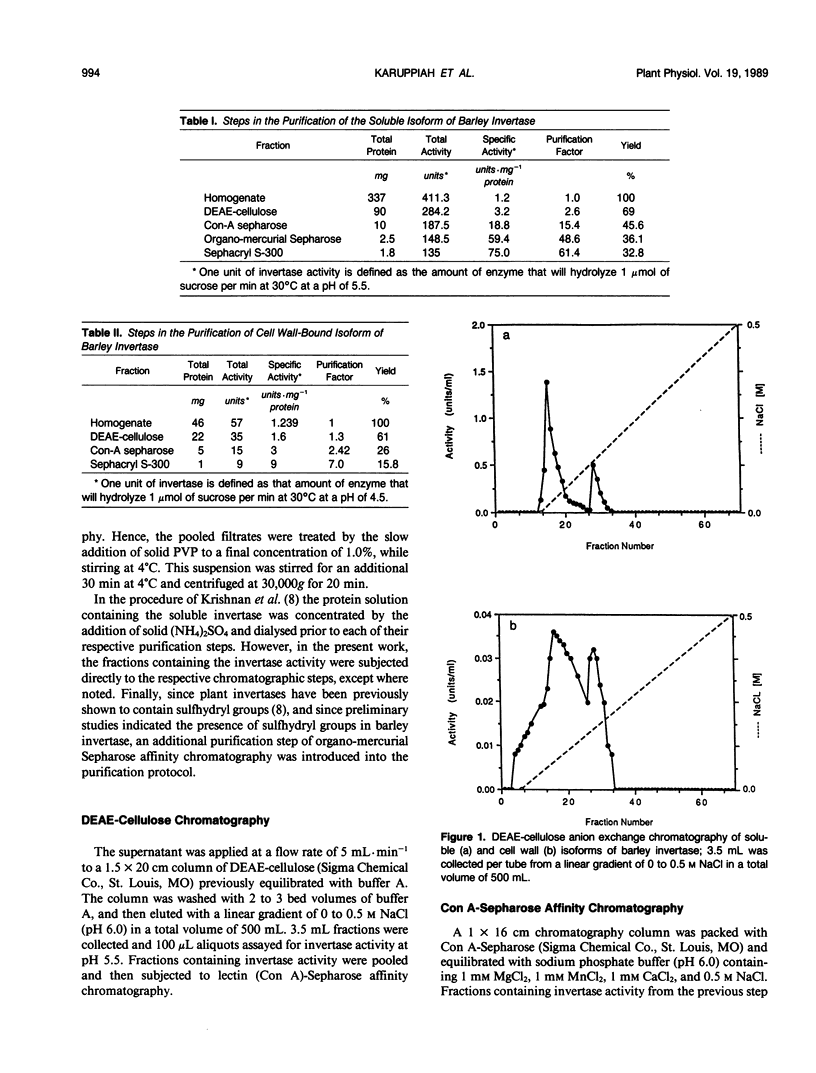
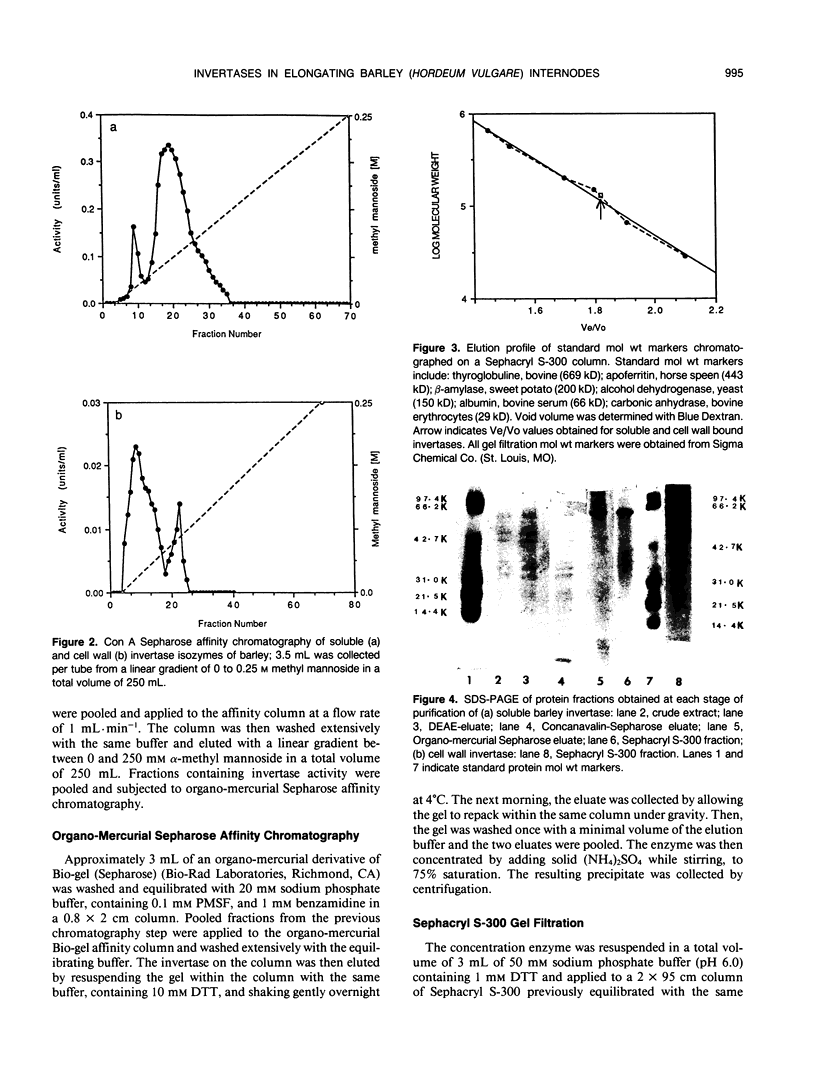
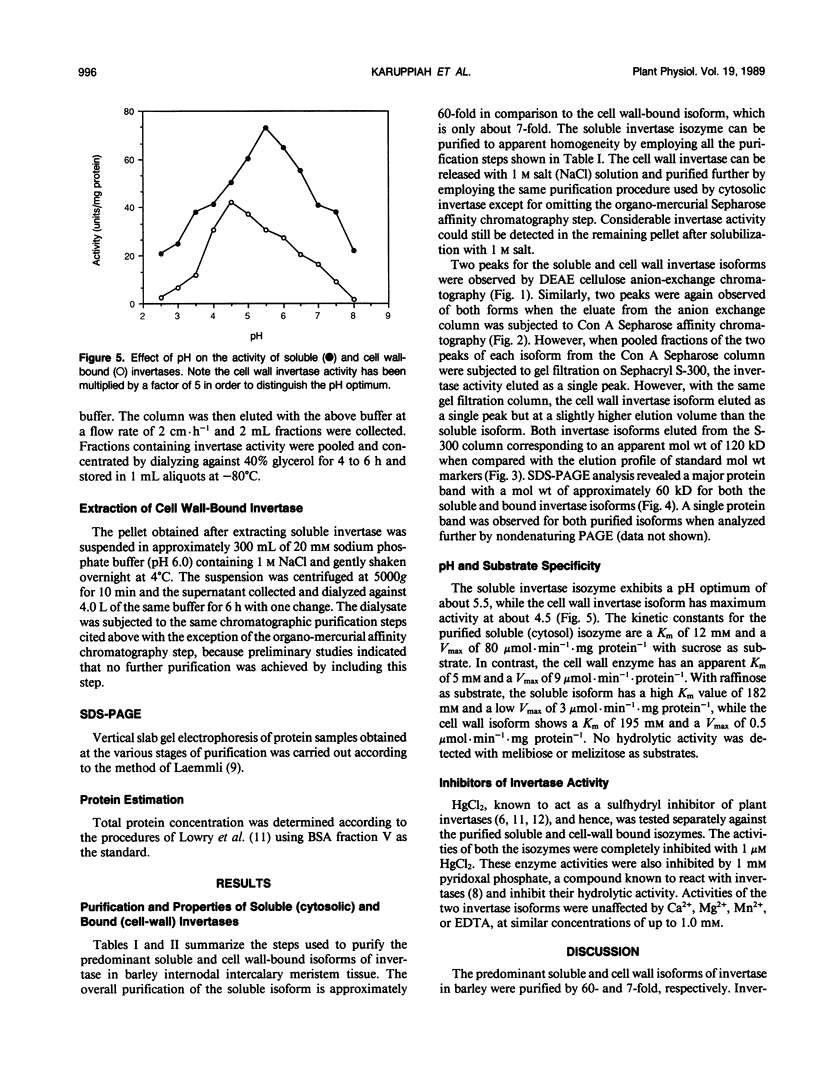
Three different isoforms of invertases have been detected in the developing internodes of barley (Hordeum vulgare). Based on substrate specificities, the isoforms have been identified to be invertases (β-fructosidases EC 3.2.1.26). The soluble (cytosolic) invertase isoform can be purified to apparent homogeneity by diethylaminoethyl cellulose, Concanavalin-A Sepharose, organomercurial Sepharose, and Sephacryl S-300 chromatography. A bound (cell wall) invertase isoform can be released by 1 molar salt and purified further by the same procedures as above except omitting the organo-mercurial Sepharose affinity chromatography step. A third isoform of invertase, which is apparently tightly associated with the cell wall, cannot be isolated yet. The soluble and bound invertase isoforms were purified by factors of 60- and 7-fold, respectively. The native enzymes have an apparent molecular weight of 120 kilodaltons as estimated by gel filtration. They have been identified to be dimers under denaturing and nondenaturing conditions. The soluble enzyme has a pH optimum of 5.5, Km of 12 millimolar, and a Vmax of 80 micromole per minute per milligram of protein compared with cell wall isozyme which has a pH optimum of 4.5, Km of millimolar, and a Vmax of 9 micromole per minute per milligram of protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hatch M. D., Sacher J. A., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. I. Studies on Enzymes of the Cycle. Plant Physiol. 1963 May;38(3):338–343. doi: 10.1104/pp.38.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P. B., Ghosheh N. S., Lacroix J. D., Soni S. L., Ikuma H. Regulation of invertase levels in Avena stem segments by gibberellic Acid, sucrose, glucose, and fructose. Plant Physiol. 1973 Sep;52(3):221–228. doi: 10.1104/pp.52.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P. B., Ghosheh N., Ikuma H. Promotion of growth and invertase activity by gibberellic Acid in developing Avena internodes. Plant Physiol. 1968 Jan;43(1):29–34. doi: 10.1104/pp.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan H. B., Blanchette J. T., Okita T. W. Wheat invertases : characterization of cell wall-bound and soluble forms. Plant Physiol. 1985 Jun;78(2):241–245. doi: 10.1104/pp.78.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Masuda H., Takahashi T., Sugawara S. Acid and alkaline invertases in suspension cultures of sugar beet cells. Plant Physiol. 1988 Jan;86(1):312–317. doi: 10.1104/pp.86.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann N. P., Lampen J. O. Purification and properties of yeast invertase. Biochemistry. 1967 Feb;6(2):468–475. doi: 10.1021/bi00854a015. [DOI] [PubMed] [Google Scholar]
- Pressey R., Avants J. K. Invertases in Oat Seedlings: SEPARATION, PROPERTIES, AND CHANGES IN ACTIVITIES IN SEEDLING SEGMENTS. Plant Physiol. 1980 Jan;65(1):136–140. doi: 10.1104/pp.65.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. B., Knox R. B. Invertases of Lilium Pollen : Characterization and Activity during In Vitro Germination. Plant Physiol. 1984 Mar;74(3):510–515. doi: 10.1104/pp.74.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]