Abstract
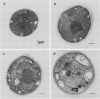
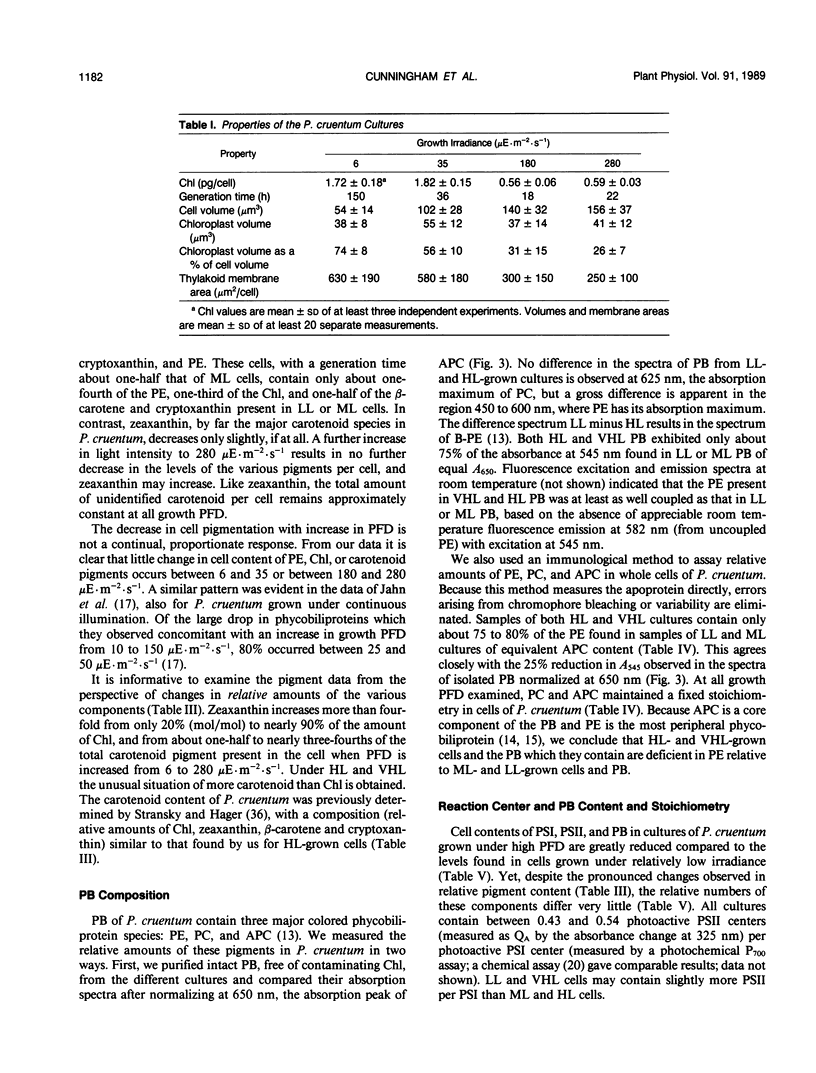
Cells of the red alga Porphyridium cruentum (ATCC 50161) exposed to increasing growth irradiance exhibited up to a three-fold reduction in photosystems I and II (PSI and PSII) and phycobilisomes but little change in the relative numbers of these components. Batch cultures of P. cruentum were grown under four photon flux densities of continuous white light; 6 (low light, LL), 35 (medium light, ML), 180 (high light, HL), and 280 (very high light, VHL) microeinsteins per square meter per second and sampled in the exponential phase of growth. Ratios of PSII to PSI ranged between 0.43 and 0.54. About three PSII centers per phycobilisome were found, regardless of growth irradiance. The phycoerythrin content of phycobilisomes decreased by about 25% for HL and VHL compared to LL and ML cultures. The unit sizes of PSI (chlorophyll/P700) and PSII (chlorophyll/QA) decreased by about 20% with increase in photon flux density from 6 to 280 microeinsteins per square meter per second. A threefold reduction in cell content of chlorophyll at the higher photon flux densities was accompanied by a twofold reduction in β-carotene, and a drastic reduction in thylakoid membrane area. Cell content of zeaxanthin, the major carotenoid in P. cruentum, did not vary with growth irradiance, suggesting a role other than light-harvesting. HL cultures had a growth rate twice that of ML, eight times that of LL, and slightly greater than that of VHL cultures. Cell volume increased threefold from LL to VHL, but volume of the single chloroplast did not change. From this study it is evident that a relatively fixed stoichiometry of PSI, PSII, and phycobilisomes is maintained in the photosynthetic apparatus of this red alga over a wide range of growth irradiance.
Full text
PDF


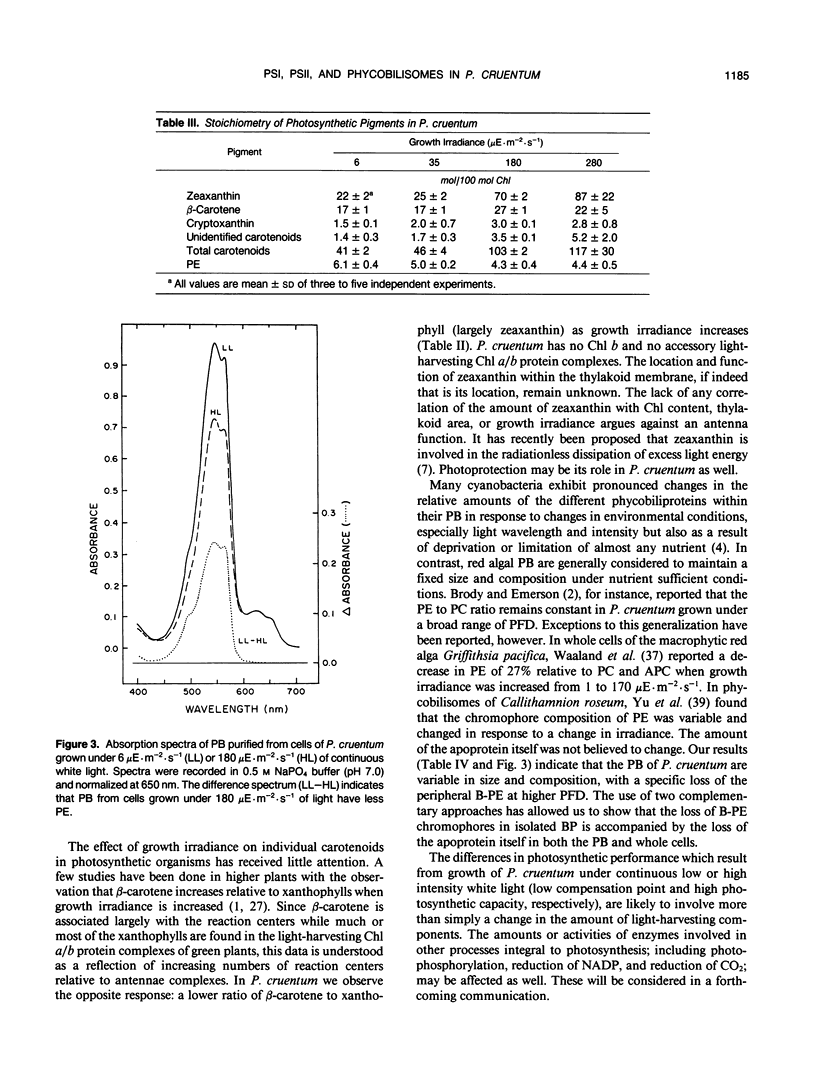


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clement-Metral J. D., Gantt E., Redlinger T. A photosystem II-phycobilisome preparation from the red alga, Porphyridium cruentum: oxygen evolution, ultrastructure, and polypeptide resolution. Arch Biochem Biophys. 1985 Apr;238(1):10–17. doi: 10.1016/0003-9861(85)90135-3. [DOI] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Zeaxanthin and the Heat Dissipation of Excess Light Energy in Nerium oleander Exposed to a Combination of High Light and Water Stress. Plant Physiol. 1988 May;87(1):17–24. doi: 10.1104/pp.87.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth M. F., Gantt E. Phycobilisome-thylakoid Topography on Photosynthetically Active Vesicles of Porphyridium cruentum. Plant Physiol. 1981 Apr;67(4):608–612. doi: 10.1104/pp.67.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner B. A., Wollman F. A. Functional Comparison of the Photosystem II Center-Antenna Complex of a Phycocyanin-less Mutant of Cyanidium caldarium with That of Chlorella pyrenoidosa. Plant Physiol. 1979 Jan;63(1):20–25. doi: 10.1104/pp.63.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski P. G., Owens T. G., Ley A. C., Mauzerall D. C. Effects of growth irradiance levels on the ratio of reaction centers in two species of marine phytoplankton. Plant Physiol. 1981 Oct;68(4):969–973. doi: 10.1104/pp.68.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A. Phycobilisomes of Porphyridium cruentum: pigment analysis. Biochemistry. 1974 Jul 2;13(14):2960–2966. doi: 10.1021/bi00711a027. [DOI] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A., Zilinskas B. Further evidence for a phycobilisome model from selective dissociation, fluorescence emission, immunoprecipitation, and electron microscopy. Biochim Biophys Acta. 1976 May 14;430(2):375–388. doi: 10.1016/0005-2728(76)90093-1. [DOI] [PubMed] [Google Scholar]
- Kursar T. A., Alberte R. S. Photosynthetic Unit Organization in a Red Alga : Relationships between Light-Harvesting Pigments and Reaction Centers. Plant Physiol. 1983 Jun;72(2):409–414. doi: 10.1104/pp.72.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. J., Whitmarsh J. Photosynthetic apparatus of pea thylakoid membranes : response to growth light intensity. Plant Physiol. 1989 Mar;89(3):932–940. doi: 10.1104/pp.89.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley A. C., Butler W. L. Effects of Chromatic Adaptation on the Photochemical Apparatus of Photosynthesis in Porphyridium cruentum. Plant Physiol. 1980 Apr;65(4):714–722. doi: 10.1104/pp.65.4.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley A. C. Effective Absorption Cross-Sections in Porphyridium cruentum: Implications for Energy Transfer between Phycobilisomes and Photosystem II Reaction Centers. Plant Physiol. 1984 Feb;74(2):451–454. doi: 10.1104/pp.74.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manodori A., Melis A. Cyanobacterial Acclimation to Photosystem I or Photosystem II Light. Plant Physiol. 1986 Sep;82(1):185–189. doi: 10.1104/pp.82.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R. Formulae for determination of chlorophyllous pigments extracted with n,n-dimethylformamide. Plant Physiol. 1982 Jun;69(6):1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Graham J. R., Wang R. T. Light Harvesting in Anacystis nidulans Studied in Pigment Mutants. Plant Physiol. 1980 Dec;66(6):1144–1149. doi: 10.1104/pp.66.6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumley F. G., Schmidt G. W. Rocket and crossed immunoelectrophoresis of proteins solubilized with sodium dodecyl sulfate. Anal Biochem. 1983 Oct 1;134(1):86–95. doi: 10.1016/0003-2697(83)90267-1. [DOI] [PubMed] [Google Scholar]
- Stransky H., Hager A. Das Carotinoidmuster und die Verbreitung des lichtinduzierten Xanthophyllcyclus in verschiedenen Algenklassen. IV. Cyanophyceae und Rhodophyceae. Arch Mikrobiol. 1970;72(1):84–96. [PubMed] [Google Scholar]
- YOCUM C. S., BLINKS L. R. Light-induced efficiency and pigment alterations in red algae. J Gen Physiol. 1958 Jul 20;41(6):1113–1117. doi: 10.1085/jgp.41.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. H., Glazer A. N., Spencer K. G., West J. A. Phycoerythrins of the Red Alga Callithamnion: VARIATION IN PHYCOERYTHROBILIN AND PHYCOUROBILIN CONTENT. Plant Physiol. 1981 Aug;68(2):482–488. doi: 10.1104/pp.68.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]