Abstract
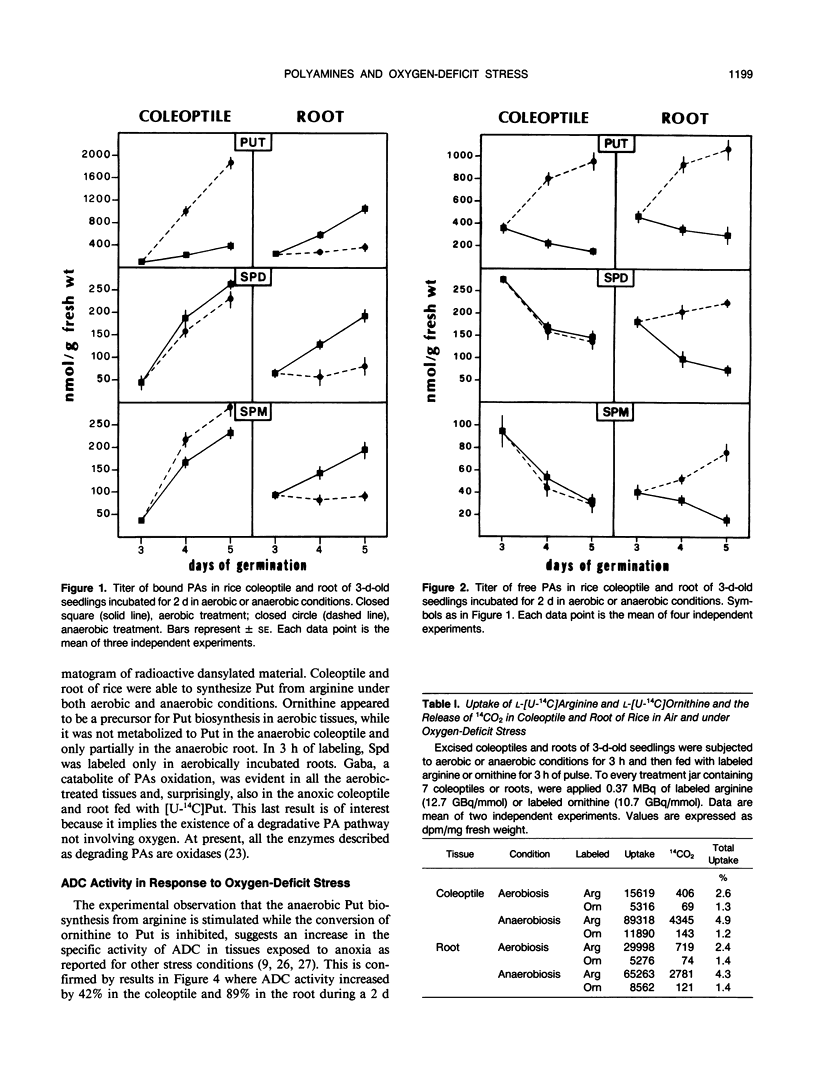
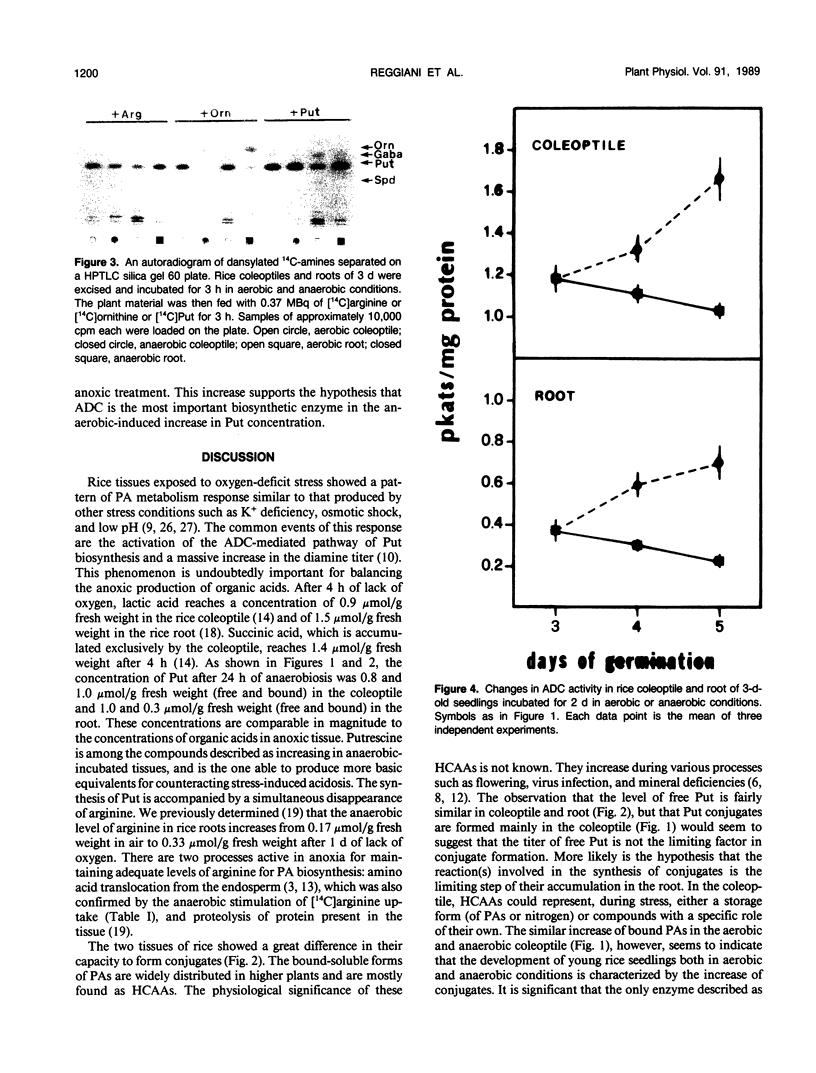
Incubation of 3-d-old seedlings of Oryza sativa L. cv Arborio under anaerobic conditions, leads to a large increase in the titer of free putrescine while aerobic incubation causes a slight decrease. After 2 days, the putrescine level is about 2.5 times greater without oxygen than in air. The rice coleoptile also accumulates a large amount of bound putrescine and, to a lesser extent, spermidine and spermine (mainly as acid-soluble conjugates). Accumulation of conjugates in the roots is severely inhibited by the anaerobic treatment. Feeding experiments with labeled amino acids showed that anoxia stimulates the release of 14CO2 from tissues fed with [14C]arginine and that arginine is the precursor in putrescine biosynthesis. After 2 d of anoxia, the activity of arginine decarboxylase was 42% and 89% greater in coleoptile and root, respectively, than in the aerobic condition. The causes of the differences in polyamine metabolism in anoxic coleoptiles and roots are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Flores H. E., Galston A. W. Polyamines and plant stress: activation of putrescine biosynthesis by osmotic shock. Science. 1982 Sep 24;217(4566):1259–1261. doi: 10.1126/science.217.4566.1259. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Andrade F. H., Anderson I. C. Further Evidence that Cytoplasmic Acidosis Is a Determinant of Flooding Intolerance in Plants. Plant Physiol. 1985 Feb;77(2):492–494. doi: 10.1104/pp.77.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Wemmer D., Walbot V., Jardetzky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]
- Young N. D., Galston A. W. Physiological control of arginine decarboxylase activity in k-deficient oat shoots. Plant Physiol. 1984 Oct;76(2):331–335. doi: 10.1104/pp.76.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. D., Galston A. W. Putrescine and Acid Stress : Induction of Arginine Decarboxylase Activity and Putrescine Accumulation by Low pH. Plant Physiol. 1983 Apr;71(4):767–771. doi: 10.1104/pp.71.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]



