Abstract
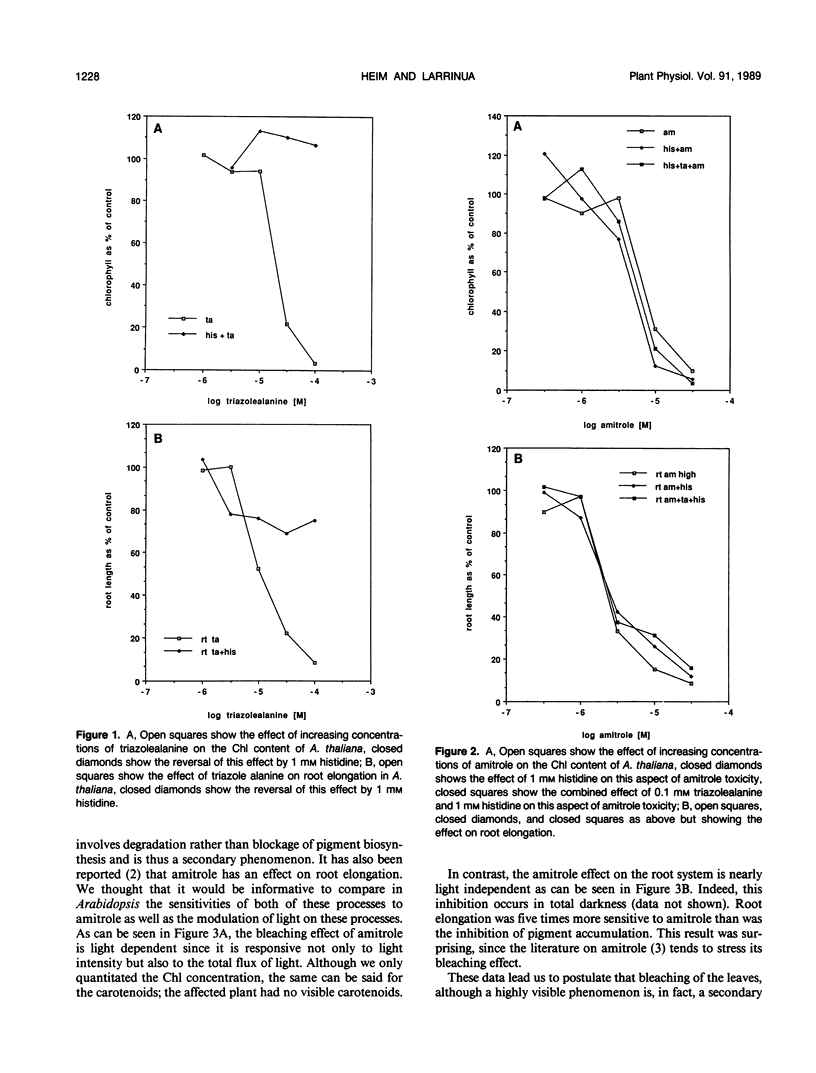
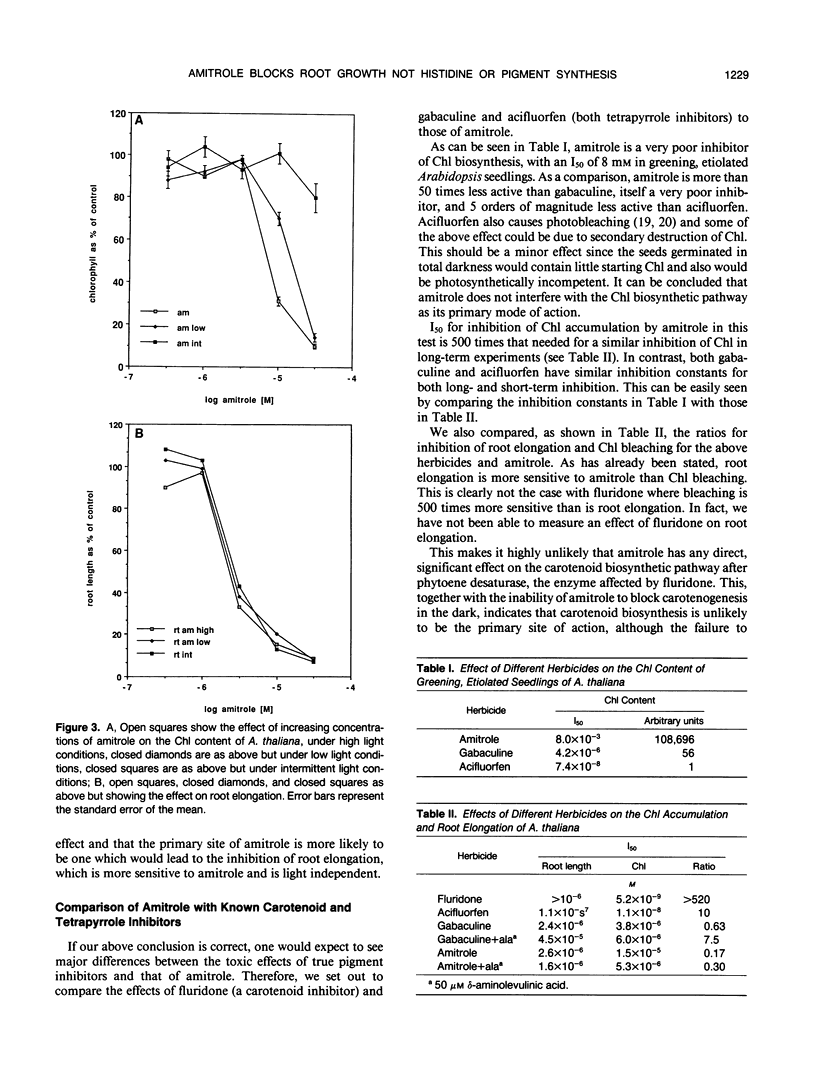
Interference with histidine metabolism, inhibition of pigment biosynthesis, or both have been the principal candidates for the primary site of action of 3-amino 1,2,4-triazole (amitrole). Arabidopsis thaliana is sensitive to 1,2,4-triazole-3-alanine, a feedback inhibitor of histidine biosynthesis, and this effect is reversed by histidine. The combination of triazolealanine and histidine, however, does not reverse the herbicidal effect of amitrole. This indicates that amitrole toxicity is not caused by histidine starvation, nor is it caused by the accumulation of a toxic intermediate of the histidine pathway. Amitrole inhibits root elongation at lower concentrations than it causes pigment bleaching in the leaves. In contrast, fluridone, a known inhibitor of the carotenoid biosynthetic pathway does not block root elongation. Fluridone also inhibits carotenoid accumulation in etiolated seedlings in the dark, but amitrole does not. Last, gabaculine and acifluorfen, but not amitrole, prevent chlorophyll accumulation in greening etiolated seedlings of Arabidopsis. These experiments cast doubt on pigment biosynthesis as the primary site of action of amitrole.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels P. G., Hyde A. Buoyant density studies of chloroplast and nuclear deoxyribonucleic acid from control and 3-amino-1,2,4-triazole-treated wheat seedlings, Triticum vulgare. Plant Physiol. 1970 Dec;46(6):825–830. doi: 10.1104/pp.46.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels P. G., Matsuda K., Siegel A., Weier T. E. Chloroplastic ribosome formation: inhibition by 3-amino-1,2,4-triazole. Plant Physiol. 1967 May;42(5):736–741. doi: 10.1104/pp.42.5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond T. J., Akers J. MECHANISM OF GROWTH INHIBITION OF ESCHERICHIA COLI BY 3-AMINO-1,2,4-TRIAZOLE. J Bacteriol. 1961 Feb;81(2):327–328. doi: 10.1128/jb.81.2.327-328.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns E. R., Buchanan G. A., Carter M. C. Inhibition of carotenoid synthesis as a mechanism of action of amitrole, dichlormate, and pyriclor. Plant Physiol. 1971 Jan;47(1):144–148. doi: 10.1104/pp.47.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörfling P., Dummler W., Mücke D. Das Auftreten von Koproperphyrin in Kulturen von Poteriochromonas stipitata nach Inkubation mit 3-Amino-1,2,4-triazol (Amitrol) Experientia. 1970;26(7):728–728. doi: 10.1007/BF02232509. [DOI] [PubMed] [Google Scholar]
- Hilton J. L., Kearney P. C., Ames B. N. Mode of action of the herbicide, 3-amino-1,2,4-triazole(amitrole): inhibition of an enzyme of histidine biosynthesis. Arch Biochem Biophys. 1965 Dec;112(3):544–547. doi: 10.1016/0003-9861(65)90093-7. [DOI] [PubMed] [Google Scholar]
- Kishore G. M., Shah D. M. Amino acid biosynthesis inhibitors as herbicides. Annu Rev Biochem. 1988;57:627–663. doi: 10.1146/annurev.bi.57.070188.003211. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A., SCHEJTER A. Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem J. 1960 Feb;74:339–348. doi: 10.1042/bj0740339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matringe M., Camadro J. M., Labbe P., Scalla R. Protoporphyrinogen oxidase as a molecular target for diphenyl ether herbicides. Biochem J. 1989 May 15;260(1):231–235. doi: 10.1042/bj2600231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matringe M., Camadro J. M., Labbe P., Scalla R. Protoporphyrinogen oxidase inhibition by three peroxidizing herbicides: oxadiazon, LS 82-556 and M&B 39279. FEBS Lett. 1989 Mar 13;245(1-2):35–38. doi: 10.1016/0014-5793(89)80186-3. [DOI] [PubMed] [Google Scholar]
- McHale N. A., Hanson K. R., Zelitch I. A Nuclear Mutation in Nicotiana sylvestris Causing a Thiamine-Reversible Defect in Synthesis of Chloroplast Pigments. Plant Physiol. 1988 Nov;88(3):930–935. doi: 10.1104/pp.88.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]


