Abstract
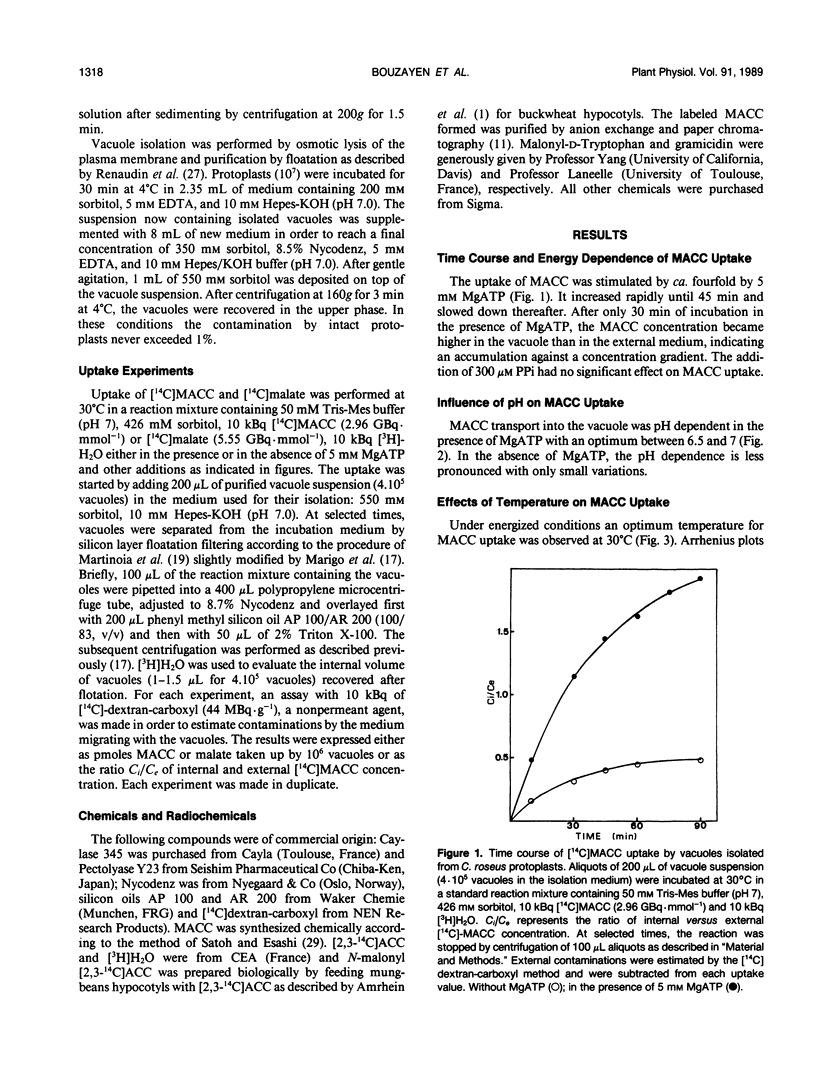
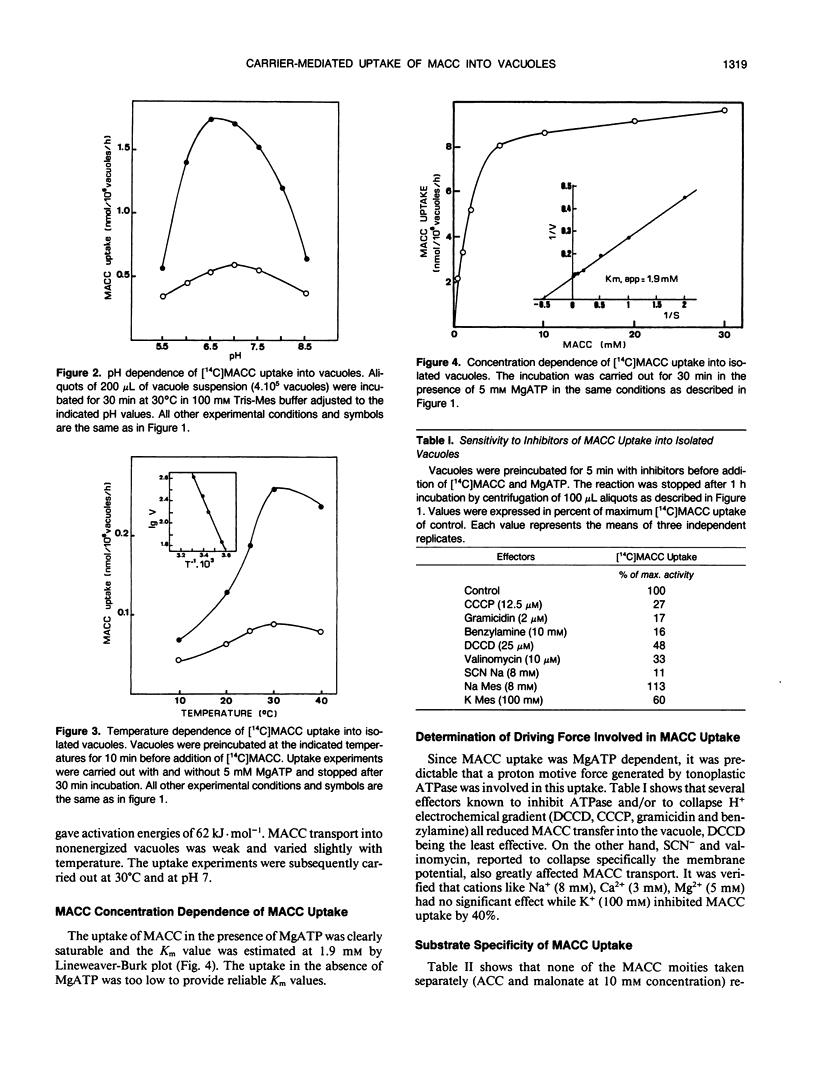
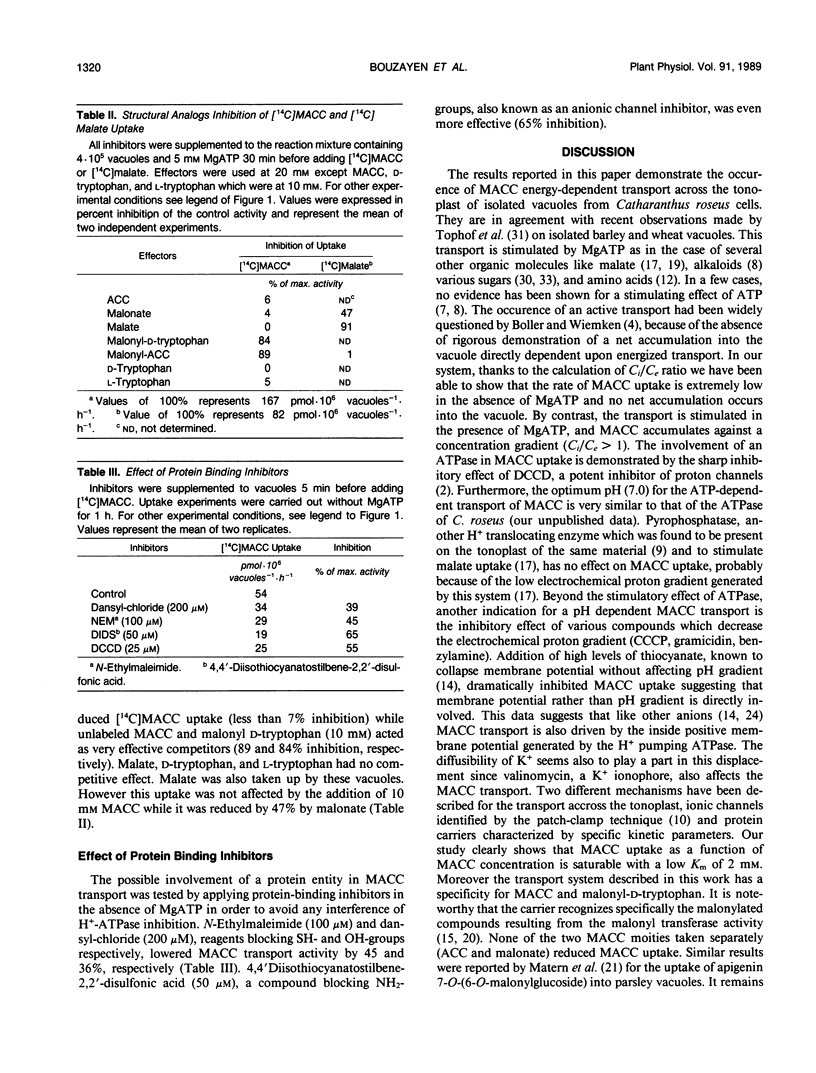
The uptake of 1-(malonylamino)cyclopropane-1-carboxylic acid (MACC), the conjugated form of the ethylene precursor, into vacuoles isolated from Catharanthus roseus cells has been studied by silicone layer floatation filtering. The transport across the tonoplast of MACC is stimulated fourfold by 5 millimolar MgATP, has a Km of about 2 millimolar, an optimum pH around 7, and an optimum temperature at 30°C. Several effectors known to inhibit ATPase (N,N′-dicyclohexylcarbodiimide) and to collapse the transtonoplastic H+ electrochemical gradient (carbonylcyanide m-chlorophenylhydrazone, gramicidin, and benzylamine) all reduced MACC uptake. Abolishing the membrane potential with SCN− and valinomycin also greatly inhibited MACC transport. Our data demonstrate that MACC accumulates in the vacuole against a concentration gradient by means of a proton motive force generated by a tonoplastic ATPase. The involvement of a protein carrier is suggested by the strong inhibition of uptake by compounds known to block SH—, OH—, and NH2— groups. MACC uptake is antagonized competitively by malonyl-d-tryptophan, indicating that the carrier also accepts malonyl-d-amino acids. Neither the moities of these compounds taken separately [1-aminocyclopropane-1-carboxylic acid, malonate, d-tryptophan or d-phenylalanine] nor malate act as inhibitors of MACC transport. The absence of inhibition of malate uptake by MACC suggests that MACC and malate are taken up by two different carriers. We propose that the carrier identified here plays an important physiological role in withdrawing from the cytosol MACC and malonyl-d-amino acids generated under stress conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Dürr M., Wiemken A. Characterization of a specific transport system for arginine in isolated yeast vacuoles. Eur J Biochem. 1975 May;54(1):81–91. doi: 10.1111/j.1432-1033.1975.tb04116.x. [DOI] [PubMed] [Google Scholar]
- Bouzayen M., Latché A., Alibert G., Pech J. C. Intracellular Sites of Synthesis and Storage of 1-(Malonylamino)cyclopropane-1-Carboxylic Acid in Acer pseudoplatanus Cells. Plant Physiol. 1988 Nov;88(3):613–617. doi: 10.1104/pp.88.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser-Suter C., Wiemken A., Matile P. A malic Acid permease in isolated vacuoles of a crassulacean Acid metabolism plant. Plant Physiol. 1982 Feb;69(2):456–459. doi: 10.1104/pp.69.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman N. E., Yang S. F., McKeon T. Identification of 1-(malonylamino) cyclopropane-1-carboxylic acid as a major conjugate of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor in higher plants. Biochem Biophys Res Commun. 1982 Jan 29;104(2):765–770. doi: 10.1016/0006-291x(82)90703-3. [DOI] [PubMed] [Google Scholar]
- Jiao X. Z., Philosoph-Hadas S., Su L. Y., Yang S. F. The Conversion of 1-(Malonylamino)cyclopropane-1-Carboxylic Acid to 1-Aminocyclopropane-1-Carboxylic Acid in Plant Tissues. Plant Physiol. 1986 Jun;81(2):637–641. doi: 10.1104/pp.81.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner K. H., Sze H. Potential-dependent anion transport in tonoplast vesicles from oat roots. Plant Physiol. 1987 Mar;83(3):483–489. doi: 10.1104/pp.83.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin B., Blasco F. Further evidence for the proton pumping work of tonoplast ATPase from Hevea latex vacuome. Biochem Biophys Res Commun. 1982 Mar 15;105(1):354–361. doi: 10.1016/s0006-291x(82)80052-1. [DOI] [PubMed] [Google Scholar]
- Matern U., Feser C., Heller W. N-malonyltransferases from peanut. Arch Biochem Biophys. 1984 Nov 15;235(1):218–227. doi: 10.1016/0003-9861(84)90271-6. [DOI] [PubMed] [Google Scholar]
- Mathieu Y., Guern J., Kurkdjian A., Manigault P., Manigault J., Zielinska T., Gillet B., Beloeil J. C., Lallemand J. Y. Regulation of Vacuolar pH of Plant Cells: I. Isolation and Properties of Vacuoles Suitable for P NMR Studies. Plant Physiol. 1989 Jan;89(1):19–26. doi: 10.1104/pp.89.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D., Krauss G., Hieke M., Gröger D. Indole Alkaloid Formation and Storage in Cell Suspension Cultures of Catharanthus roseus. Planta Med. 1983 May;48(1):20–23. doi: 10.1055/s-2007-969871. [DOI] [PubMed] [Google Scholar]
- Rosa N., Neish A. C. Formation and occurrence of N-malonylphenylalanine and related compounds in plants. Can J Biochem. 1968 Aug;46(8):799–806. [PubMed] [Google Scholar]


