Abstract
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Following initial infection of airway epithelia, SARS-CoV-2 invades a wide range of cells in multiple organs, including pancreatic islet cells. Diabetes is now recognized as a risk factor for severe COVID-19 outcomes, including hospitalisation and death. Additionally, COVID-19 is associated with higher risk of new-onset diabetes and metabolic complications of diabetes. One mechanism by which these deleterious outcomes may occur is via destruction of insulin-producing islet β cells, either directly by SARS-CoV-2 entry into β cells or indirectly due to inflammation and fibrosis in the surrounding microenvironment. While the canonical pathway of viral entry via angiotensin converting enzyme 2 (ACE2) has been established as a major route of SARS-CoV-2 infection in the lung, it may not be solely responsible for viral entry into the endocrine pancreas. This is likely due to divergent expression of viral entry factors amongst different tissues. For example, expression of ACE2 has not been unequivocally demonstrated in β cells. Thus, it is important to understand how other proteins known to be highly expressed in pancreatic endocrine cells may be involved in SARS-CoV-2 entry, with the view that these could be targeted to prevent demise of the β cell in COVID-19. To that end, this review discusses alternate receptors of SARS-CoV-2 (CD147 and GRP78), as well as mediators (furin, TMPRSS2, cathepsin L, ADAM17, neuropilin-1 and heparan sulphate) that may facilitate SARS-CoV-2 entry into pancreatic islets independent of or in conjunction with ACE2.
Keywords: COVID-19, SARS-CoV-2, islet, ACE2, diabetes, furin, TMPRSS2, cathepsin L, ADAM17, GRP78, NRP1, CD147, heparan sulfate
Introduction
COVID-19 and Diabetes
In 2019, 9.3% of the world’s population was estimated to have diabetes, a number that is predicted to increase by 25% within the next ten years (Saeedi et al. 2019). At the same time, despite availability of effective vaccines, SARS-CoV-2 infections are predicted to continue around the world (Telenti et al. 2021). These two pandemics are closely linked, and each condition may contribute to the global burden of the other. Prevalent diabetes is an independent risk factor for hospitalisation and death among individuals with COVID-19 (Wander et al. 2021). Conversely, a positive test for SARS-CoV-2 is associated with a 40% higher risk of new-onset diabetes and increased use of glucose-lowering medications, including insulin (Al-Aly et al. 2021; Wander et al. 2022; Xie & Al-Aly 2022). Moreover, an excess burden of metabolic complications commonly associated with diabetes is observed within months of COVID-19 infection. These sequelae include disorders of lipid metabolism and obesity (Al-Aly et al. 2021). Such outcomes are exacerbated in hospitalised individuals with COVID-19, where risks are highest in those admitted to intensive care (Al-Aly et al. 2021).
While islet cell autoantibodies are rarely detected, new-onset diabetes after COVID-19 appears to be frequently complicated by diabetic ketoacidosis (DKA) (Misra et al. 2021). New-onset diabetes that presents with DKA has been recognized as ketosis-prone and is marked by significant impairment of insulin secretion and insulin action at presentation (Vellanki & Umpierrez 2017). Together, these lines of evidence suggest that early β-cell injury may contribute to the pathogenesis of new-onset diabetes after COVID-19; however, mechanisms contributing to such β-cell injury, including the critical pathways mediating viral entry, remain poorly characterised.
SARS-CoV-2 Enters Human Pancreatic Endocrine Cells
Studies investigating viral entry into islet α and β cells have done so using either SARS-CoV-2 itself, or a pseudovirus where the envelope glycoprotein is replaced by the SARS-CoV-2 spike protein. In human islets, SARS-CoV-2 was shown to enter α and β cells ex vivo (Yang et al. 2020; Müller et al. 2021), with viral infiltrates detected mostly in insulin-positive β cells (Wu et al. 2021). Although considered functionally immature, human pluripotent stem cell-derived α and β cells were permissive to SARS-CoV-2 pseudovirus infection in vitro and in vivo when xenografted into mice (Yang et al. 2020). Further, immunohistochemical and molecular analyses showed SARS-CoV-2 infiltration in pancreas from humans with COVID-19, particularly in β cells, sometimes more so than in other pancreatic endocrine cells (Müller et al. 2021; Steenblock et al. 2021; Tang et al. 2021a; Wu et al. 2021). In sum, there is convincing evidence that SARS-CoV-2 directly infects the islet.
Following infection, SARS-CoV-2 causes morphological, transcriptional, and functional derangements in islet cells (Mine et al. 2022). For example, dilatation and vacuolization of the endoplasmic reticulum (ER)–Golgi apparatus complex was evident in SARS-CoV-2-infected islet cells (Müller et al. 2021). At the transcriptional level, loss of β-cell identity was observed, wherein α- and acinar cell markers were increased and insulin was decreased in SARS-CoV-2- vs. mock-infected β cells (Müller et al. 2021; Tang et al. 2021a). Insulin protein was also decreased in SARS-CoV-2-infected β cells (Tang et al. 2021a), as was number of insulin secretory granules, C-peptide and insulin immunoreactivity, and glucose-stimulated insulin secretion (Müller et al. 2021). It is suggested these perturbations may be mediated by ER stress (Müller et al. 2021). SARS-CoV-2 infection of islets also upregulated several interferon-stimulated genes (Müller et al. 2021), indicative of an inflammatory response that may exacerbate cellular damage.
ACE2-mediated SARS-CoV-2 Entry
Angiotensin converting enzyme 2 (ACE2) is the canonical receptor of SARS-CoV-2. Following binding of SARS-CoV-2 to ACE2, there are two routes for viral entry into host cells: direct membrane fusion and endocytosis.
Host cell membrane fusion pathway
SARS-CoV-2 contains a transmembrane spike glycoprotein comprising the S1 and S2 subunits. S1 binds to the host cell receptor and S2 mediates fusion of the viral and host cell membranes. As illustrated in Fig. 1, SARS-CoV-2 entry begins with binding of the spike protein to ACE2 (Hoffmann et al. 2020a). SARS-CoV-2-spike must be sequentially cleaved along the S1/S2 junction, usually by furin (Hoffmann et al. 2020b), and at a second site (S2’) by host serine proteases, typically transmembrane serine protease 2 (TMPRSS2) (Hoffmann et al. 2020a) (Fig. 1). This exposes hydrophobic amino acid residues in processed S2 that embed themselves into the host cell membrane, further facilitating fusion of the viral envelope and host cell membrane (Huang et al. 2020) (Fig. 1).
Figure 1:
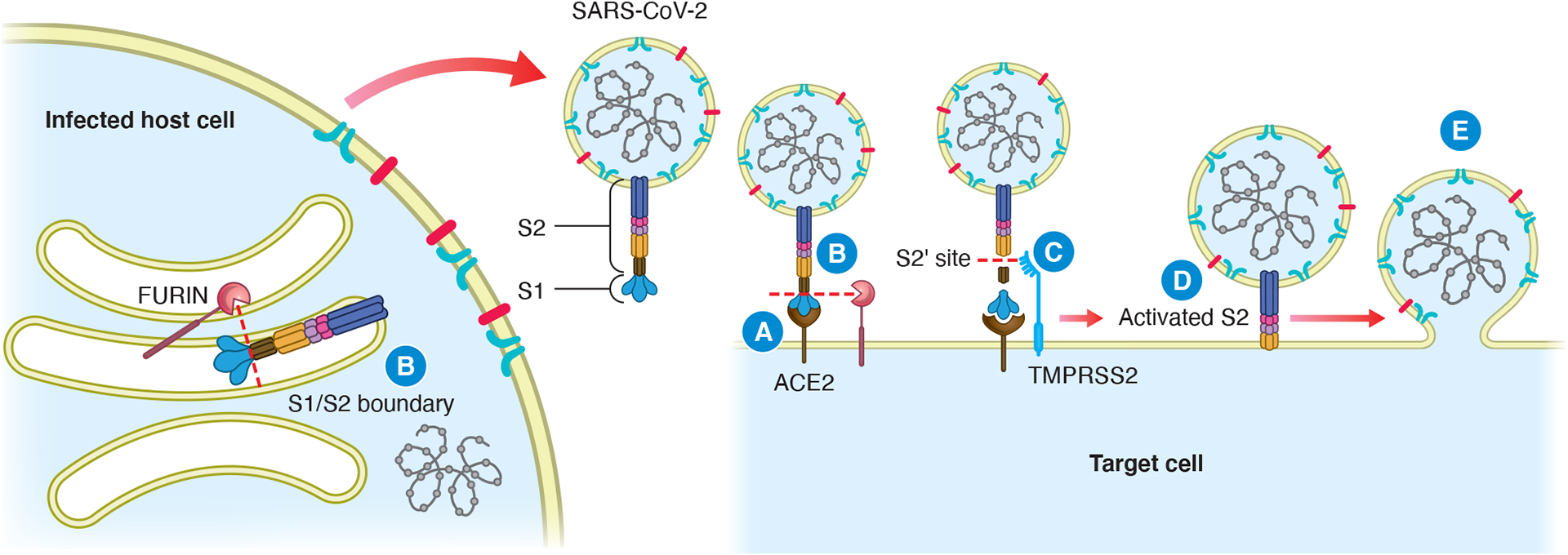
ACE2-mediated SARS-CoV-2 viral entry via the membrane fusion pathway. SARS-CoV-2-spike binds to ACE2 on the target cell surface (step A), after which furin cleaves SARS-CoV-2-spike at the S1/S2 boundary (step B). Furin may also cleave the spike protein at the S1/S2 boundary during viral production, prior to virus release into the extracellular space. TMPRSS2 cleaves the S2 protein at the S2’ site (step C), which allows the insertion of hydrophobic amino acid residues in the activated S2 subunit into the plasma membrane (step D), facilitating membrane fusion between the viral envelope and the host cell plasma membrane (step E).
Alternative endocytosis pathway
Alternatively, SARS-CoV-2-spike binding to ACE2 is followed by uptake of virions into endosomes. Cathepsin L (CTSL), a pH-sensitive endosomal protease, primes the spike by cleaving it into smaller fragments after the initial furin-mediated S1/S2 cleavage (Zhao et al. 2021). The viral envelope then fuses with the endosomal membrane, releasing viral machinery and genetic material.
Although the membrane fusion pathway is 100–1000 times more efficient than the endocytosis pathway (as measured for SARS-CoV) (Matsuyama et al. 2005), the mode of viral entry amongst different cells is dependent on protease expression (Padmanabhan et al. 2020).
ACE2 Expression in α and β Cells
While the literature is largely in agreement that SARS-CoV-2 enters the islet, the mechanism of viral entry is still being debated. A major point of uncertainty is whether ACE2 is expressed in α and β cells (El-Huneidi et al. 2021). Data in this regard have derived mostly from RNA-sequencing (RNA-seq) and immunohistochemistry analyses.
RNA-sequencing
Two studies demonstrated ACE2 expression in α and β cells, one via single-cell RNA-seq (scRNA-seq) on adult human islets (Yang et al. 2020), and the other by analysing existing scRNA-seq datasets (Lazartigues et al. 2020). However, most of the data from a total of 11 bulk and single-cell RNA-seq datasets show little ACE2 expression in α or β cells (Coate et al. 2020; Kusmartseva et al. 2020; Lee et al. 2020; Liu et al. 2020; Qadir et al. 2021; Wu et al. 2021) – and quantitatively much lower expression than key genes enriched in α (e.g. GCG, ARX, IRX2) and β (e.g. INS, PDX1, MAFA) cells (Coate et al. 2020).
Immunohistochemistry
Among studies showing positive ACE2 staining in islets, there is general agreement that β cells express more ACE2 than α cells. In fact, β cells had the highest frequency of ACE2 staining among pancreatic endocrine cell types (Müller et al. 2021). One paper described islet ACE2 immunofluorescence as weak and diffuse, arising from a subset of cells that were identified to be β cells and not α cells (Fignani et al. 2020). Further, ACE2 in β cells was predominantly found in insulin secretory granules, with some expression on the plasma membrane (Fignani et al. 2020). Interestingly, a study that utilized various antibodies raised against different regions of ACE2 found that the prevalent ACE2 isoform in β cells is short-ACE2 (Fignani et al. 2020). Given that short-ACE2 lacks the amino acid residues required to bind SARS-CoV-2-spike (Onabajo et al. 2020), it is unlikely to be responsible for viral entry. Finally, two larger cohort studies utilising multiple anti-ACE2 antibodies on pancreatic sections from female and male donors with and without SARS-CoV-2 infection and a range of ages and BMIs found that pancreatic endocrine cells exhibited little/no ACE2 expression (Coate et al. 2020; Kusmartseva et al. 2020). Despite extensive validation, the commonly used anti-ACE2 antibodies produce vastly different staining patterns amongst various studies (Yang et al. 2010; Brar et al. 2017; Coate et al. 2020; Fignani et al. 2020; Hikmet et al. 2020; Kusmartseva et al. 2020; Lazartigues et al. 2020; Yang et al. 2020; Müller et al. 2021; Qadir et al. 2021; Steenblock et al. 2021; Wu et al. 2021). These differences could not be explained by β-cell maturity level or technical aspects, like antibody dilution (Coate et al. 2020).
What may be contributing to the variability in islet ACE2 expression across studies of human donors? One factor may be differences in clinical characteristics such as age, sex, and presence of SARS-CoV-2 infection. Another important factor, particularly in SARS-CoV-2 positive donors, is presence of pro-inflammatory cytokines, which can upregulate β-cell/islet ACE2 expression (Fignani et al. 2020). Since cytokine levels change over the course of COVID-19 disease, the timing of sample collection in relation to onset of illness in SARS-CoV-2 positive donors is a critical consideration for assessing ACE2 expression.
The cytokine milieu to which islets are exposed may influence the ability of ACE2 to mediate SARS-CoV-2 entry. In Fig. 2, potential scenarios are presented for SARS-CoV-2 entry into islet cells in the setting of high vs. low ACE2 expression, taking into account the roles of other viral entry mediators. When ACE2 is sufficiently expressed, cofactors and co-receptors may assist ACE2-mediated entry, and alternative receptors may work in parallel to increase the rate of viral entry. If islet ACE2 levels remain too low to permit viral entry, alternative receptors may be primarily responsible for SARS-CoV-2 entry into islet cells. Additionally, viral invasion of the islet may induce a local inflammatory response (Müller et al. 2021). This may affect expression of entry mediators (Chu et al. 2018; Cantuti-Castelvetri et al. 2020), potentially altering the mode of subsequent infection by virions into the same or surrounding host cells so that it differs from that of the original infection.
Figure 2:
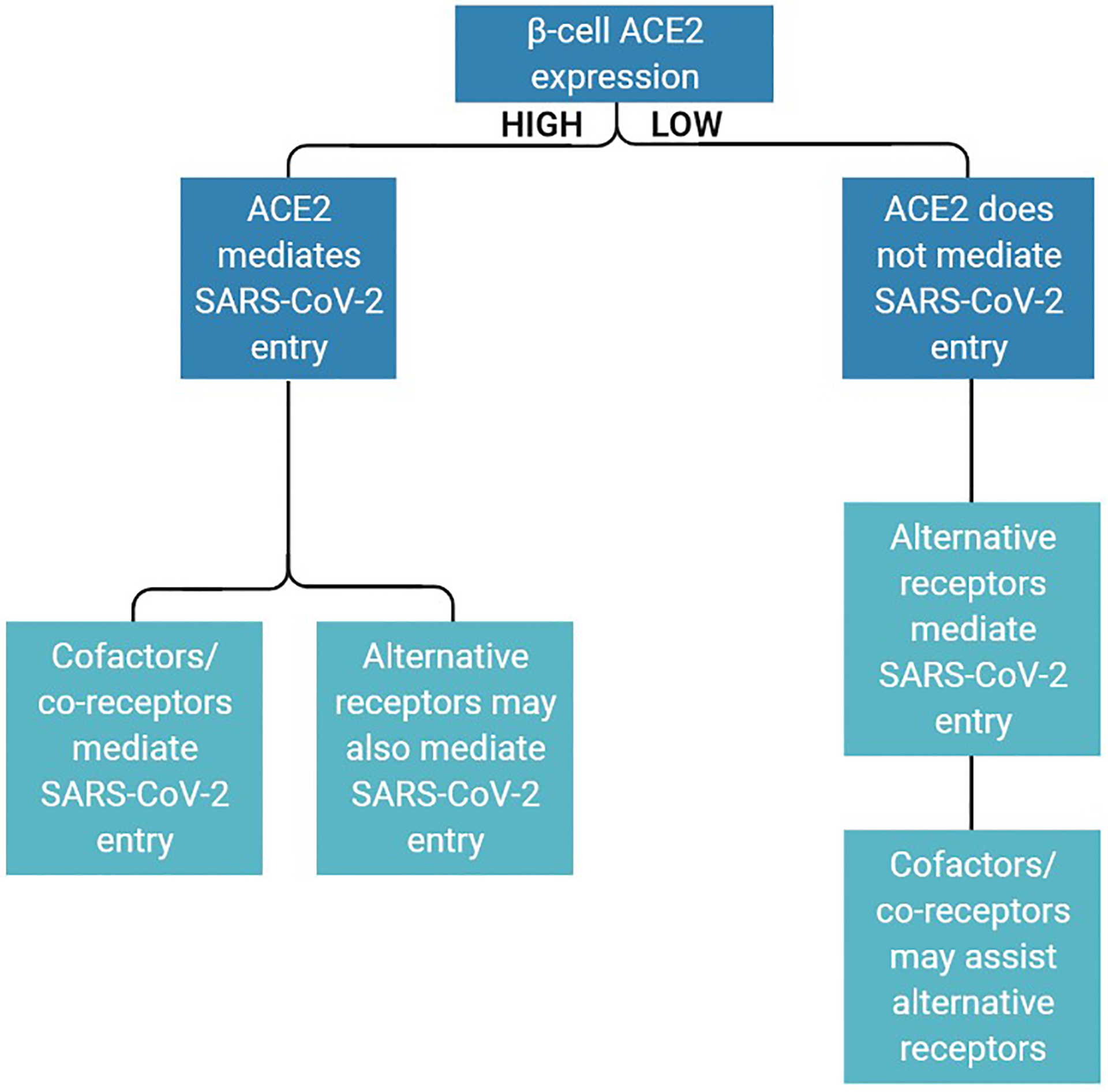
Potential involvement of SARS-CoV-2 entry factors in islet endocrine cells under conditions of high or low ACE2 expression. ACE2 serves as the predominant SARS-CoV-2 receptor when its levels are sufficiently high for viral entry, and/or its expression is induced by pro-inflammatory cytokines. Under these circumstances, cofactors/co-receptors assist ACE2, and co-receptors further allow viral entry. If ACE2 levels are too low to permit SARS-CoV-2 entry, alternative receptors facilitate viral entry, with assistance from cofactors/co-receptors.
The following sections outline the roles of different viral entry mediators, which may function as, or alongside, SARS-CoV-2 receptors (Fig. 3). Thus far, many studies of SARS-CoV-2 entry have utilized lung tissue or related models; this review aims to contextualise data on entry mediators to the endocrine pancreas.
Figure 3:
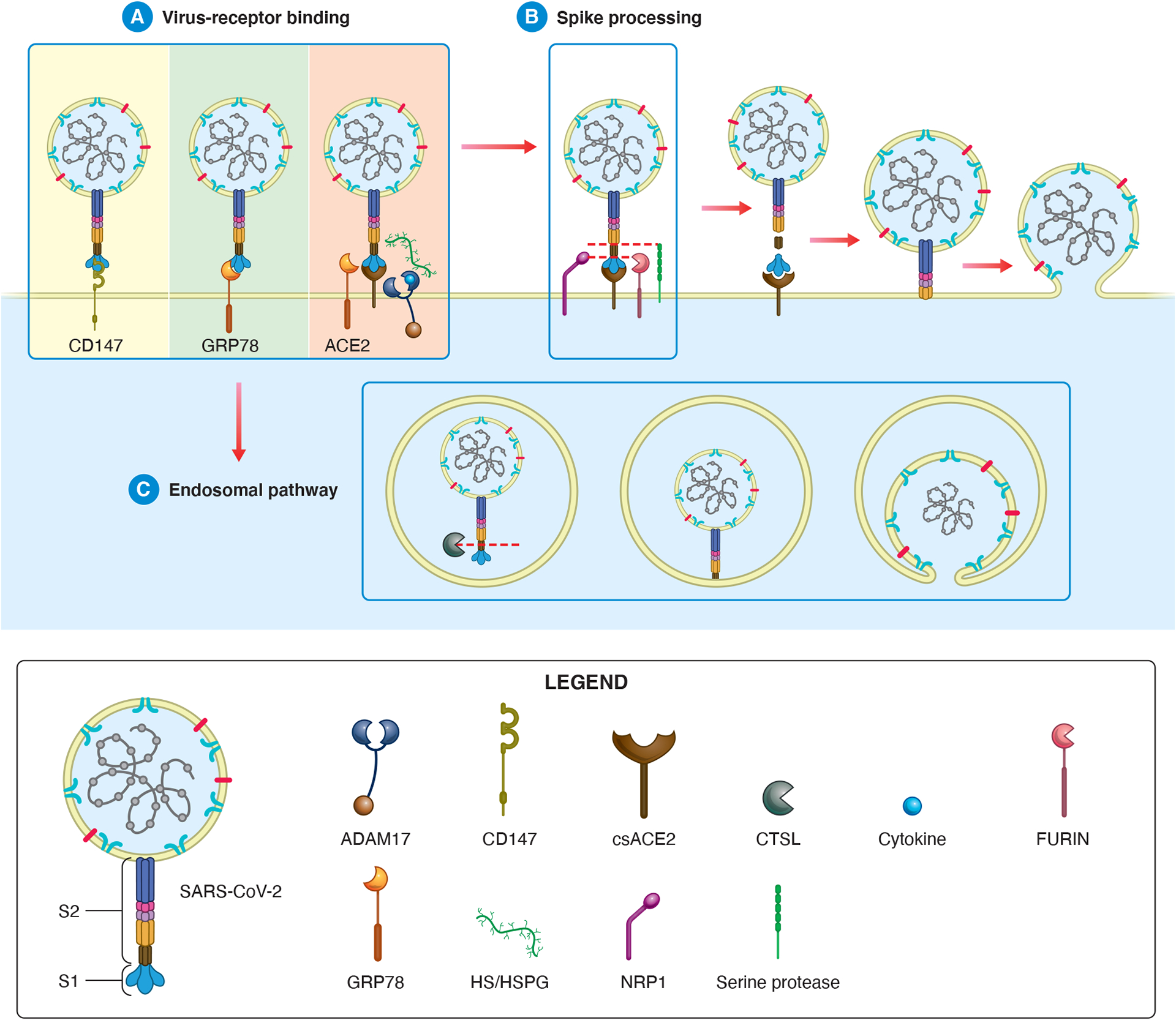
Proposed roles for alternative receptors and mediators of viral entry into β cells based on studies in other cell types. (A) The mature virion binds to its host cell receptor, which may be CD147 (left), GRP78 (centre) or ACE2 (right). If the host cell receptor is ACE2, SARS-CoV-2-spike-ACE2 binding may be facilitated by GRP78 or HS/HSPG. ADAM17 may be involved in ACE2-mediated SARS-CoV-2 entry in several ways. On one hand, ADAM17 cleaves csACE2, releasing sACE2, which can impede SARS-CoV-2-receptor binding. On the other hand, ADAM17 can cleave and activate cytokines for release, which may upregulate viral entry mediators. On the whole, the net impact of ADAM17 on viral entry is unclear. (B) Before membrane fusion, the spike protein must be processed. After S1/S2 cleavage by furin (which can also occur during viral production), NRP1 can stabilise the SARS-Cov-2-S1-CendR motif to increase the rate of viral spike processing. S2’ cleavage may occur by another host protease in the absence of TMPRSS2. (C) Alternatively, rather than viral envelope-host cell membrane fusion, the SARS-CoV-2-receptor complex can be taken up via receptor-mediated endocytosis. CTSL in the endosome cleaves SARS-CoV-2-spike at a site distinct from the S1/S2 boundary and S2’ site. Viral entry is complete when the viral envelope fuses with the host cell surface membrane or endosomal membrane.
Alternative SARS-CoV-2 Receptors
Cluster of differentiation 147
Function
Cluster of differentiation 147 (CD147), also known as basigin or extracellular matrix metalloprotease inducer, is a widely expressed member of the immunoglobulin superfamily. It is predominantly membrane bound and highly glycosylated. It is thought to be involved in cell-cell recognition, although its role in inducing extracellular matrix metalloproteases is most extensively studied. CD147 was found to be increased in pulmonary fibrosis (Guillot et al. 2006), and inhibition of CD147 reduced differentiation of fibroblasts to myofibroblasts (Ulrich & Pillat 2020). This raises questions about its possible role in islet fibrosis, especially with COVID-19.
Expression in the islet
CD147 is very highly expressed in α, β, and γ cells (Uhlén et al. 2015; Segerstolpe et al. 2016), though one study reported it absent in islets (Zhao et al. 2001). Though not reported in islets, CD147 is upregulated in various cell types upon ER (Grass & Toole 2016) and oxidative (Ke et al. 2012) stress, which occur in patients with COVID-19 (Rosa-Fernandes et al. 2021).
Role in SARS-CoV-2 infection
The ability of CD147 to serve as an alternative SARS-CoV-2 receptor is exemplified by the demonstration that cells otherwise not susceptible to viral infection allowed SARS-CoV-2 (and pseudovirus) entry upon CD147 expression (Wang et al. 2020). Additionally, humanised CD147 mice infected with SARS-CoV-2 had detectable viral loads in their lungs, unlike virus-infected wild-type mice (Wang et al. 2020; Geng et al. 2021). Some (Wang et al. 2020; Geng et al. 2021), but not all (Ragotte et al. 2021; Shilts et al. 2021), studies provide evidence that SARS-CoV-2-spike binds to CD147. CD147-SARS-CoV-2-spike binding is also supported by molecular modelling data (Helal et al. 2020), and evidence of CD147 and SARS-CoV-2-spike colocalization in lung and kidney tissue from donors with COVID-19 (Wang et al. 2020). Further, pseudovirus infection of CD147-expressing cells could be neutralized by addition of the extracellular domain of CD147, suggesting it competes with membrane-bound CD147 for spike binding (Wang et al. 2020). In intervention studies, CD147 overexpression increased SARS-CoV-2 infection (Wang et al. 2020), whereas CD147 blockade/knockdown had the opposite effect in most (Wang et al. 2020; Fenizia et al. 2021; Geng et al. 2021), but not all (Ragotte et al. 2021) studies.
CD147 facilitates SARS-CoV-2 entry into host cells via endocytosis (Wang et al. 2020). It does not bind ACE2 (Wang et al. 2020); however, CD147 silencing reduced ACE2 protein levels (Fenizia et al. 2021), suggesting an interaction between the two proteins that is yet to be understood. After SARS-CoV-2 infection, CD147 was upregulated in human airway epithelial cells (Fenizia et al. 2021)— a response that may exacerbate virus entry.
Concluding remarks
CD147 may serve as an alternative SARS-CoV-2 receptor (Fig. 3A)—as opposed to a cofactor/co-receptor, especially as it promotes SARS-CoV-2 infection under conditions in which (human) ACE2 receptor is not expressed. It may also act via other mechanisms to permit viral infection. CD147 is worthy of study in the mediation of SARS-CoV-2 entry in islet cells with and without high ACE2 expression.
Glucose Regulatory Protein 78
Function
Glucose regulatory protein 78 (GRP78) is an ER-localised chaperone that regulates ER signalling molecules to ensure proper protein folding. During ER stress, the unfolded protein response upregulates GRP78. However, GRP78 recycling from the Golgi system to the ER becomes saturated and GRP78 is missorted to the cell surface. Thus, cell surface GRP78 (csGRP78) can act as a receptor and may be involved in signal transduction (Ibrahim et al. 2019). Although ER and plasma membrane GRP78 are most commonly studied, GRP78 also exists as mitochondrial, cytosolic, secreted, and nuclear proteins (Ni et al. 2011).
Expression in the islet
GRP78 is expressed in all pancreatic cell types, including β cells (Uhlén et al. 2015; Segerstolpe et al. 2016). Some (Allagnat et al. 2012; Brozzi et al. 2015), but not all (Cardozo et al. 2005; Pirot et al. 2006; Åkerfeldt et al. 2008) studies show GRP78 to be upregulated in β cells under cytokine stress. Further, GRP78 is increased in diabetic mouse models (Laybutt et al. 2007; Wang et al. 2009) and islets from donors with type 2 diabetes (T2D) compared to non-diabetic donors (Laybutt et al. 2007; Hull et al. 2009). However, some studies find no significant increase in islet GRP78 levels in T2D donors (Marchetti et al. 2007; Marselli et al. 2020).
Role in SARS-CoV-2 infection
GRP78 may promote viral infection by serving as an alternative receptor and/or stabilising spike-receptor binding ((Ha et al. 2020); Fig. 3A). In silico modelling predicted that GRP78 docks to the receptor-binding domain of SARS-CoV-2-spike (Ibrahim et al. 2020), which has the same GRP78 recognition site as other human and bat coronaviruses (Elfiky 2020). GRP78 binds MERS-CoV-spike in vitro, and blockade/knockdown of GRP78 reduced MERS-CoV infection (Chu et al. 2018). Additionally, GRP78 overexpression increased, but was not sufficient for, MERS-CoV entry into cells (Chu et al. 2018). Similar to studies of MERS-CoV, GRP78 can bind SARS-CoV-2 and ACE2 in vitro at the ER and cell surface of kidney epithelial cells (Carlos et al. 2021). Specifically depleting or blocking csGRP78 decreased SARS-CoV-2 and pseudovirus entry (Carlos et al. 2021). Hence, csGRP78 may bind SARS-CoV-2-spike and act as an alternative receptor, cofactor or co-receptor, working in place of or in conjunction with ACE2 to facilitate viral entry.
SARS-CoV-2 infection induces ER stress in host cells (Köseler et al. 2020; Rosa-Fernandes et al. 2021), likely due to an inability to accommodate the drastic increase in protein production that occurs during viral replication (Aoe 2020). Hence, coronavirus infection typically upregulates GRP78 (Chan et al. 2006; Chu et al. 2018). In line with this, serum GRP78 levels are increased in COVID-19 patients compared to both COVID-19-negative pneumonia patients and healthy volunteers (Sabirli et al. 2021). Under conditions of ER stress, other SARS-CoV-2 entry factors can also modulate GRP78 levels. For example, CD147 mediates ER stress-induced GRP78 upregulation (Tang et al. 2012). Thus, CD147 upregulation following ER stress may exacerbate GRP78 mediation of SARS-CoV-2 entry in a feed-forward fashion. Additionally, GRP78 can impact cellular ACE2 levels. GRP78 knockdown decreased cell surface ACE2 (csACE2) (but not total ACE2) independent of ER stress, suggesting that GRP78 may be involved in ACE2 trafficking (Carlos et al. 2021). Thus, via several interactions with other entry mediators, GRP78 can influence SARS-COV-2 entry.
Concluding remarks
GRP78 can promote SARS-CoV-2 entry via multiple mechanisms, which may be due in part to its relationship with other viral entry factors. Apart from ER and cell surface GRP78, other GRP78 forms may also support viral infection, necessitating study of their interactions with SARS-CoV-2 and viral entry mediators. Given its high expression in islets, GRP78 is a prime candidate for initiating and/or mediating viral invasion of pancreatic endocrine cells.
Cofactors and Co-receptors in SARS-CoV-2 Entry
Several proteins outlined below work alongside ACE2 to mediate SARS-CoV-2 entry in various cell types. Cofactors furin, TMPRSS2, and CTSL are proteases that prime SARS-CoV-2-spike for entry; ADAM17 exerts proteolytic activity on ACE2; co-receptors neuropilin-1 (NRP1) and heparan sulfate (HS) assist SARS-CoV-2-spike-ACE2 binding. According to existing literature, the primary role of these mediators is to increase the efficiency of ACE2-mediated viral entry. This may make ACE2 a significant contributor to SARS-CoV-2 infection in β cells. Similarly, and what represents a gap in current knowledge, is that these mediators may also work alongside alternative receptors to permit viral entry into islet cells.
Furin
Function
Furin is a transmembrane endoprotease that cleaves a diverse range of proproteins prior to secretion, as part of their maturation process (Thomas 2002). It is localised to the trans-Golgi network, but also cycles between the cell surface and endosomes (Shapiro et al. 1997; Thomas 2002). In islet cells, furin controls proliferation and differentiation (Kayo et al. 1996), as well as secretory granule acidification (Louagie et al. 2008), the latter being critical for β-cell granule maturation and proinsulin-to-insulin conversion.
Expression in the islet
Furin has moderate to high expression in human pancreatic endocrine cells (Uhlén et al. 2015; Brouwers et al. 2021), including β cells (Sawada et al. 2000; Segerstolpe et al. 2016; Tang et al. 2021a), as shown by RNA-seq and immunohistochemistry.
Role in SARS-CoV-2 infection
As a proprotein convertase, furin cleaves and activates many viral proteins (including SARS-CoV-2 proteins (Murgolo et al. 2021)), thereby facilitating viral entry into host cells (Fig. 1; Fig. 3B). This cleavage may occur during viral production in an infected cell, or on virus entry into a host cell (Hoffmann et al. 2020b; Shang et al. 2020; Papa et al. 2021). SARS-CoV-2-spike contains a polybasic furin cleavage site (FCS) at the S1/S2 junction (Coutard et al. 2020), which is absent in other SARS coronaviruses (Coutard et al. 2020). Studies in other viruses show that insertion of a similar polybasic FCS increases virulence (Claas et al. 1998). Interestingly, the structure of the FCS suggests that SARS-CoV-2 uses molecular mimicry to hijack host cell machinery for viral entry (Anand et al. 2020). In vitro studies showed that furin knockout decreased viral production 100-fold (Papa et al. 2021). Mutants of SARS-CoV-2-spike resistant to S1/S2 cleavage had significantly reduced entry into TMPRSS2-positive but not TMPRSS2-negative cells, suggesting that furin-mediated cleavage may only be relevant in cells where the membrane fusion pathway predominates over the endocytosis pathway (Hoffmann et al. 2020b). Cells with diminished furin activity still exhibited SARS-CoV-2 spike cleavage, albeit at much reduced levels (Papa et al. 2021). This is likely attributed to less efficient spike processing by other cellular proteases, such as trypsin, matriptase, and proprotein convertase 1 (Jaimes et al. 2020)—of which the last is highly expressed in β cells and thus may be relevant for viral entry in islets.
Concluding remarks
The unique furin cleavage site in SARS-CoV-2 makes viral envelope-host cell membrane fusion more efficient. Since furin is highly expressed in islets, studies are needed to confirm furin-mediated S1/S2 cleavage in islet cells. Moreover, other islet proteases/proprotein convertases may work alongside furin to cleave the SARS-CoV-2 spike protein at the S1/S2 junction (Jaimes et al. 2020; Tang et al. 2021b), potentially increasing the rate of viral entry.
Transmembrane Serine Protease 2
Function
TMPRSS2 is a membrane-anchored serine protease first characterized as being androgen-regulated in the prostate. It plays an important role in prostate cancer development and progression (Chen et al. 2010), but has also been associated with various processes including digestion, tissue remodeling, blood coagulation, fertility and inflammation.
Expression in the islet
TMPRSS2 expression in islets remains somewhat unclear. Moderate-to-high TMPRSS2 expression in human islets was found by immunostaining (Steenblock et al. 2021), and corroborated by RNA-seq and microarray analysis (Taneera et al. 2020); however, specific cell type was not reported in these studies. Another study showed TMPRSS2 to be highly expressed in a subset of pancreatic endocrine cells enriched in α, β and δ cells (Uhlén et al. 2015), while yet another found TMPRSS2 expression in α but not β cells (Segerstolpe et al. 2016). In contrast, scRNA-seq of primary human islets found low TMPRSS2 levels in α and β cells (Yang et al. 2020). Integrated analysis of six RNA-seq databases found that less than 1.5% of α and β cells expressed TMPRSS2 (Coate et al. 2020). However, another analysis of five scRNA-seq datasets found that 17% of α and 5.7% of β cells expressed TMPRSS2 (Kusmartseva et al. 2020). Overall, TMPRSS2 expression seems to be moderately low in α and β cells, but likely high enough in other islet cell types to contribute to moderate/high expression in the islet as a whole.
Role in SARS-CoV-2 infection
Once SARS-CoV-2-spike is bound to ACE2, TMPRSS2 cleaves the spike protein at the S2’ site to facilitate SARS-CoV-2 entry into cells via the membrane fusion pathway (Hoffmann et al. 2020a) (Fig. 1). TMPRSS2 can also cleave ACE2 at its cytoplasmic tail, increasing viral uptake through the CTSL/endocytosis pathway (Heurich et al. 2014). TMPRSS2 inhibitors were shown to hamper SARS-CoV-2 viral entry. Camostat mesylate, which inhibits serine proteases like TMPRSS2, reduced SARS-CoV-2-spike entry into TMPRSS2-positive cells, but not TMPRSS2-negative cells (Hoffmann et al. 2020a). Interestingly, camostat mesylate combined with E-64d (CTSL inhibitor) prohibited viral entry into TMPRSS2-positive cells, whereas E-64d alone did not (Hoffmann et al. 2020a). Overall, this suggests that TMPRSS2 expression can compensate for low CTSL activity in SARS-CoV-2-spike processing (Hoffmann et al. 2020a). In cells with low/no TMPRSS2 expression, other TMPRSS2-related proteases may cleave SARS-CoV-2-spike (Hoffmann et al. 2021). One such protease is TMPRSS4, whose gene expression is highly correlated with ACE2 in lung cells, even more so than TMPRSS2 (Wruck & Adjaye 2020). TMPRSS4 has been shown to promote SARS-CoV-2 entry in intestinal enterocytes by cleaving SARS-CoV-2-spike (Zang et al. 2020). Within the pancreas, TMPRSS4 expression seems to be limited to exocrine and endothelial cell types (Segerstolpe et al. 2016; Coate et al. 2020; Kusmartseva et al. 2020; Lee et al. 2020), though it is upregulated in pancreatic cancer cells (Katopodis et al. 2021). It is therefore possible that TMPRSS4 is involved in mediating SARS-CoV-2 infection in both the exocrine and endocrine pancreas, the latter via islet endothelial cell interactions.
Concluding remarks
The proteolytic activity of TMPRSS2 is important for SARS-CoV-2 viral entry in many cell types, though it is unknown whether this holds true if the entry receptor is not ACE2. Although TMPRSS2 expression in α and β cells may be moderate/low, compensation by other cellular proteases like CTSL may explain permissiveness of α and β cells to SARS-CoV-2 infection.
Cathepsin L
Function
CTSL is a lysosomal cysteine endoprotease, though it is also found in the nucleus and can be secreted. It plays a major role in degrading extracellular, cytoplasmic and nuclear proteins, and is involved in a diverse range of processes such as autophagy, apoptosis, cell cycle regulation, bone resorption, antigen processing, and tumor invasion/metastasis (Yadati et al. 2020). It is required for the development of type 1 diabetes (T1D) in mouse models (Maehr et al. 2005), and regulates human and mouse islet cell proliferation (Lo et al. 2019).
Expression in the islet
CTSL is ubiquitously expressed (Dana & Pathak 2020), consistent with its wide range of cellular functions. It is present in all pancreatic cell types (Segerstolpe et al. 2016; Tang et al. 2021a) and is moderately expressed in pancreatic endocrine cells (Tang et al. 2021a), including α and β cells (Kusmartseva et al. 2020). β-cell CTSL expression in T2D donors was shown to be higher than in non-diabetic donors (Kusmartseva et al. 2020).
Role in SARS-CoV-2 infection
In a human hepatoma cell line (Huh7), CTSL levels were elevated following SARS-CoV-2 pseudovirus entry (Zhao et al. 2021), while CTSL overexpression per se increased pseudovirus entry (Zhao et al. 2021). Conversely, CTSL knockdown in Huh7 cells or pharmacological inhibition in humanised ACE2 mice using E64d decreased SARS-CoV-2 pseudovirus entry (Zhao et al. 2021). Similarly, inactivation of CTSL with ammonium chloride reduced SARS-CoV-2 entry, but inhibition was weaker in TMPRSS2-positive versus TMPRSS2-negative cells (Hoffmann et al. 2020a). This indicates that TMPRSS2 and CTSL can each compensate for inactivity of the other.
CTSL cleaves SARS-CoV-2-spike downstream of the S1/S2 site, at a region distinct from the TMPRSS2 cleavage site (Murgolo et al. 2021) (Fig. 3C). This action of CTSL enhances virus entry (Zhao et al. 2021). S1/S2 cleavage site mutations (ΔCS) only reduced SARS-CoV-2 entry in cells utilising the ACE2-TMPRSS2 pathway (Hoffmann et al. 2020b). SARS-COV-2-ΔCS replicates faster than wild-type SARS-CoV-2 in cells lacking TMPRSS2 (Peacock et al. 2021; Zhu et al. 2021), reinforcing a role for CTSL in increased virulence under permissive conditions.
CTSL can be secreted, often under systemic or local inflammatory conditions (Gomes et al. 2020). In keeping with this, COVID-19 patients had elevated circulating CTSL levels compared to healthy volunteers, positively correlating with disease severity (Zhao et al. 2021). This suggests that circulating CTSL may exacerbate viral entry during inflammation that accompanies COVID-19.
Concluding remarks
SARS-CoV-2 infection and CTSL seem to have a bidirectional relationship, whereby viral infection increases CTSL levels and in turn, CTSL mediates SARS-CoV-2 entry. While largely studied in the context of ACE2, this protease may work similarly with alternative receptors, and is a good candidate for mediating viral entry into pancreatic endocrine cells with little/no TMPRSS2 expression.
A Disintegrin and Metalloprotease 17
Function
A disintegrin and metalloprotease 17 (ADAM17), also known as tumour necrosis factor α converting enzyme, is a membrane anchored protein responsible for proteolysis of several cell surface proteins (including ACE2; Fig. 3A) to enable ‘shedding’ of their ectodomains.
Expression in the islet
ADAM17 is expressed in the islet, including β cells. A subset of pancreatic endocrine cells enriched in α, β, and δ cells shows moderate ADAM17 expression (Uhlén et al. 2015). Similarly, in α- and β-cell populations from non-diabetic and/or T2D donors, ADAM17 is expressed moderately (Segerstolpe et al. 2016; Coate et al. 2020). Furthermore, circulating ADAM17 levels were elevated in patients with COVID-19 (Palacios et al. 2021).
Role in SARS-CoV-2 infection
In the case of SARS-CoV, internalisation of the virus-ACE2 complex upregulates ADAM17 expression/activity (Haga et al. 2008), as does ER stress (Rzymski et al. 2012). GRP78, which is also upregulated under cytokine/ER stress, protects ADAM17 from inhibition by protein disulphide isomerases (Schäfer et al. 2017). The increase in ADAM17 activity enhances ACE2 ‘shedding’, which decreases csACE2 (Kuba et al. 2005). This may explain the higher soluble ACE2 (sACE2) levels in COVID-19 patients compared to healthy controls (van Lier et al. 2021). Thus, shedding may be a cellular defence mechanism, whereby spike-csACE2 binding is reduced (Zipeto et al. 2020). However, SARS-CoV-2-sACE2 can enter cells via endocytosis mediated by angiotensin II type 1 receptor (AT1R) or vasopressin receptor 1B (AVPR1B) (Yeung et al. 2021), though this has yet to be studied in pancreatic endocrine cells.
While not yet shown with SARS-CoV-2, ADAM17 inhibition significantly attenuated SARS-CoV entry in vitro, and reduced viral titres in mice in vivo (Haga et al. 2010). This appears to conflict with the idea above, where ADAM17 upregulation would reduce viral entry into cells. One explanation is that ADAM17 upregulation may increase the cleavage and maturation of several cytokines (Zipeto et al. 2020; Schreiber et al. 2021), whose increased levels may upregulate mediators of SARS-CoV-2 entry.
Concluding remarks
While ADAM17 may be capable of either decreasing or increasing SARS-CoV-2 entry into cells, it is unclear which effect predominates. Schreiber et. al reviews ADAM17’s possible roles in COVID-19 infection, including entry and post-infection inflammation-mediated damage (Schreiber et al. 2021). In sum, more studies are needed to better understand how ADAM17 affects SARS-CoV-2 infection of the islet.
Neuropilin-1
Function
NRP1 is a cell surface receptor involved in angiogenesis and organ development. It exists in two isoforms—one is truncated and secreted (sNRP1), and the other is a transmembrane protein (Gagnon et al. 2000). NRP1 has a large extracellular domain that binds ligands in a host of signalling pathways associated with cell migration, growth, and development (Pellet-Many et al. 2008). While its cytoplasmic domain has no known signalling sequence, literature on NRP1’s capability to signal independently is divided (Pellet-Many et al. 2008). Interestingly, minor alleles of NRP1 were associated with T1D in children (Hasan et al. 2010).
Expression in the islet
NRP1 is highly expressed in islets (Tang et al. 2021a; Wu et al. 2021). Within the pancreas, its expression is mostly confined to islets, especially β cells (Hasan et al. 2010), but rarely α cells (Hasan et al. 2010; Wu et al. 2021). NRP1 was found to be upregulated in β cells from humans with COVID-19 (Wu et al. 2021).
Role in SARS-CoV-2 infection
Furin-mediated SARS-CoV-2-spike cleavage creates a C-terminal motif (known as CendR) on S1 to which NRP1 binds (Cantuti-Castelvetri et al. 2020; Daly et al. 2020; Li & Buck 2021). NRP1 stabilises the C-terminus of S1, facilitating more efficient S1/S2 cleavage (Fig. 3B), and allowing S2 to mediate membrane fusion more rapidly (Li & Buck 2021). Specifically inhibiting binding between NRP1 and SARS-CoV-2-S1-CendR reduces viral infection in various cell types, including human islets (Cantuti-Castelvetri et al. 2020; Daly et al. 2020; Wu et al. 2021)
When infected with SARS-CoV-2, NRP1-negative ACE2-positive HeLa cells exhibited less viral entry than cells expressing both NRP1 and ACE2, but cells lacking ACE2 exhibited no viral entry (Daly et al. 2020). In human islets infected with SARS-CoV-2 ex vivo, scRNA-seq showed that ACE2- and NRP1-positive cells had more SARS-CoV-2-nucleocapsid transcripts than cells expressing either ACE2 or NRP1 (Tang et al. 2021a). ACE2-positive NRP1-negative cells had fewer transcripts than ACE2-negative NRP1-positive cells, and double-negative cells showed little/no infection (Tang et al. 2021a). Further, SARS-CoV-2-spike pseudovirus entry was independently facilitated by ACE2 but not TMPRSS2 or NRP1 (Cantuti-Castelvetri et al. 2020). Together, these data support a role for NRP1 as a co-receptor that potentiates ACE2-mediated SARS-CoV-2 infection (Kyrou et al. 2021), while data on its ability to independently permit viral entry is limited.
Concluding remarks
NRP1 is highly expressed in β cells, where it is upregulated in patients with COVID-19 and may serve to exacerbate infection. Its inhibition in human islets reduced SARS-CoV-2 infection, making it a good therapeutic drug target. While NRP1 facilitates SARS-CoV-2 entry as a co-receptor with ACE2 in various cell types, it may function similarly with alternative receptors that are present in islets – this is an area of investigation that requires further study.
Heparan sulfate
Function
HS exists as a highly acidic, negatively charged, unbranched polysaccharide and is either conjugated to proteins (HS proteoglycans; HSPGs) or remains unconjugated. Present in all cell types, both HS and HSPGs act as cell surface co-receptors, altering ligand binding affinity. They also maintain extracellular matrix structure, thereby playing a role in cell-cell adhesion and angiogenesis. In mouse islets, HS was shown to be important for β-cell maturation and insulin secretion (Takahashi et al. 2009), as well as β-cell survival and protection from autoimmunity and T1D (Ziolkowski et al. 2012).
Expression in the islet
HS exhibits high expression in β cells and islets (Ziolkowski et al. 2012). Mouse models of T2D had lower islet HS and HSPG levels than wild-type mice (Dhounchak et al. 2021).
Role in SARS-CoV-2 infection
HS binds SARS-CoV-2-spike (Clausen et al. 2020) through its receptor-binding domain (Clausen et al. 2020). This increases proximity of SARS-CoV-2 to the host cell membrane. The heparan-binding site on SARS-CoV-2-spike is adjacent to, but distinct from, the ACE2-binding residues (Clausen et al. 2020). Hence, SARS-CoV-2-spike may bind both ACE2 and HS simultaneously (Clausen et al. 2020) (Fig. 3B). After binding to HS, the receptor-binding domain of SARS-CoV-2-spike undergoes a conformational change (Mycroft-West et al. 2020), which may increase the number of spike proteins bound to any one ACE2 receptor (Clausen et al. 2020).
Treatment with heparinases (Clausen et al. 2020) and disruption of genes involved in HSPG biosynthesis (Clausen et al. 2020; Zhang et al. 2020) markedly reduced SARS-CoV-2 viral infection rates, without impacting ACE2 levels (Clausen et al. 2020) or cell viability (Clausen et al. 2020; Zhang et al. 2020). HS-assisted viral entry requires the actin cytoskeleton, consistent with HS-assisted endocytosis (Zhang et al. 2020).
Concluding remarks
HS is highly expressed in β cells/islets, where it could mediate SARS-CoV-2 infection as a co-receptor. There is evidence that SARS-CoV-2 entry is enhanced by formation of the ACE2-SARS-CoV-2-HS complex. Since HS does not directly bind ACE2, it is a good candidate for studies investigating SARS-CoV-2 recruitment to the cell surface when alternative receptors are involved.
Other mediators of interest
Several other proteins may mediate SARS-CoV-2 entry in cells that do not utilise the ACE2/TMPRSS2 pathway. One such protein is transferrin receptor (TFRC), a cell surface receptor responsible for endocytosis of iron. TFRC is highly expressed in islets, and is present in β cells (Segerstolpe et al. 2016; Wu et al. 2021). TFRC directly binds SARS-CoV-2-spike (Tang et al. 2020), and blocking this binding significantly reduces viral entry (Tang et al. 2020). Since TFRC shuttles between the cytoplasm and plasma membrane, it may transport SARS-CoV-2 in a similar fashion (Tang et al. 2020).
Dipeptidyl peptidase-4 (DPP-4), along with being the MERS-CoV receptor, is a target for several T2D medications. While DPP-4 is expressed in the islet, there is evidence to both support (Bugliani et al. 2018; Steenblock et al. 2021) and refute (Omar et al. 2014; Segerstolpe et al. 2016) its expression in β cells. Some (Li et al. 2020; Vankadari & Wilce 2020), but not all (Cameron et al. 2021) in silico studies find DPP-4 to bind SARS-CoV-2-S1. Further, one in vitro study observed no DPP-4–SARS-CoV-2-spike binding (Xi et al. 2020). DPP-4 was found to be increased in airway samples from patients with COVID-19 (Amati et al. 2020; Maremanda et al. 2020), and a meta-analysis reported that its inhibition was associated with lower COVID-19 mortality (Yang et al. 2021), though this idea faces some resistance (Noh et al. 2021). Overall, more studies are needed to determine DPP-4’s role in SARS-CoV-2 entry.
Other molecules involved in endocytosis, like PIKfyve (Baranov et al. 2020) and TPC2 (Grimm & Tang 2020), may be useful targets to reduce SARS-CoV-2 entry into cells. HDL scavenger receptor B type 1 has been shown to facilitate ACE2-mediated SARS-CoV-2 entry (Wei et al. 2020). Additionally, as previously mentioned, SARS-CoV-2–sACE2 can enter cells via receptor-mediated endocytosis using AT1R and/or AVPR1B. The relevance and impact of these proteins on viral entry in islet cells requires investigation.
Mediators as Pharmacological Targets
Several therapeutic agents have been proposed and/or approved for treating COVID-19. Here, we highlight two examples that specifically target SARS-CoV-2 entry mediators, and we contrast their potential utility in limiting COVID-19 infection of pancreatic endocrine cells.
Meplazumab is a humanised anti-CD147 monoclonal antibody. In vitro studies demonstrated its efficacy in blocking SARS-CoV-2 replication in Vero E6 cells and SARS-CoV-2-pseudovirus entry in human T cells (Wang et al. 2020). Several clinical trials are underway to test its effectiveness against COVID-19 infection in humans. In one of these trials, meplazumab was found to reduce disease severity and accelerate recovery, especially in severe and critical COVID-19 cases (Bian et al. 2021). Given that CD147 is highly expressed in islet endocrine cells, meplazumab may prove useful in reducing entry of SARS-CoV-2 and its deleterious effects in the islet.
Another drug of interest is nafamostat mesylate, which exhibits anti-inflammatory and anticoagulant properties and is currently approved to treat pancreatitis. Nafamostat mesylate is a serine protease inhibitor active against TMPRSS2 (Hempel et al. 2021). In a screen of 24 FDA-approved drugs, it was found to exhibit the greatest antiviral efficacy against SARS‐CoV‐2 in a human lung cell line whose susceptibility to virus entry is largely TMPRSS2‐dependent (Ko et al. 2021). Nafamostat mesylate has also been shown to reduce SARS-CoV-2 infection in mouse models (Li et al. 2021). Despite these promising pre-clinical data, results in humans with COVID-19 infection have been discouraging. For example, there was no significant difference in time to clinical improvement (Zhuravel et al. 2021) or in-hospital outcomes (Inokuchi et al. 2021) between hospitalised patients with COVID-19 treated with versus without nafamostat. Another study reported no evidence of anti-inflammatory, anticoagulant or antiviral activity with nafamostat (Quinn et al. 2022). In considering these human data together with evidence that TMPRSS2 expression is moderately low in islet α and β cells, nafamostat mesylate may not be a good candidate for reducing entry of SARS-CoV-2 and its deleterious effects in the islet.
Conclusions
SARS-CoV-2 infects pancreatic endocrine cells, including β cells. The canonical ACE2-TMPRSS2 pathway (Fig. 1) has been extensively researched in airway epithelia, but it remains to be fully understood in the islet. Given that islet ACE2 and TMPRSS2 expression is uncertain, investigation into alternative mediators, including their interactions with one another, is needed to better understand mechanisms underlying SARS-CoV-2 infection of islets.
Many of the mediators discussed exhibit moderate to high expressions in the islet. Given their islet expression patterns coupled with their role in other cells, we propose that furin, CTSL, GRP78, NRP1, CD147 and heparan sulphate are likely mediators of SARS-CoV-2 entry in the endocrine pancreas (Fig. 3). Data on CD147 suggest that it may be an alternative receptor for SARS-CoV-2 entry. GRP78 has been shown to mediate SARS-CoV-2 entry; however, more study is needed to deduce its dependence on other receptors. Furin, CTSL, NRP1 and heparan sulphate may assist ACE2 and/or the dominant SARS-CoV-2 entry receptor in the islet to increase viral entry but are unlikely to facilitate viral entry independently. For ADAM17, more work is needed to resolve whether its upregulation after viral entry promotes or hinders infection by subsequent virions. Lastly, while TMPRSS2 mediates viral entry in other cell types, it does not seem to be adequately expressed in β cells for this function.
With respect to islet ACE2, its induction by pro-inflammatory cytokines (and ER stress) may make it an important contributor to islet SARS-CoV-2 entry (Fig. 2), including exacerbation of infection via feed-forward mechanisms. To better understand SARS-CoV-2 entry in the context of cytokine and ER stress, a potential avenue for further study is the ACE2/ADAM17/GRP78 interplay. In Fig. 4, we propose a model for how ACE2, GRP78, ADAM17 and CD147 may interact under conditions of ER stress, including the inflammatory response that accompanies COVID-19.
Figure 4:
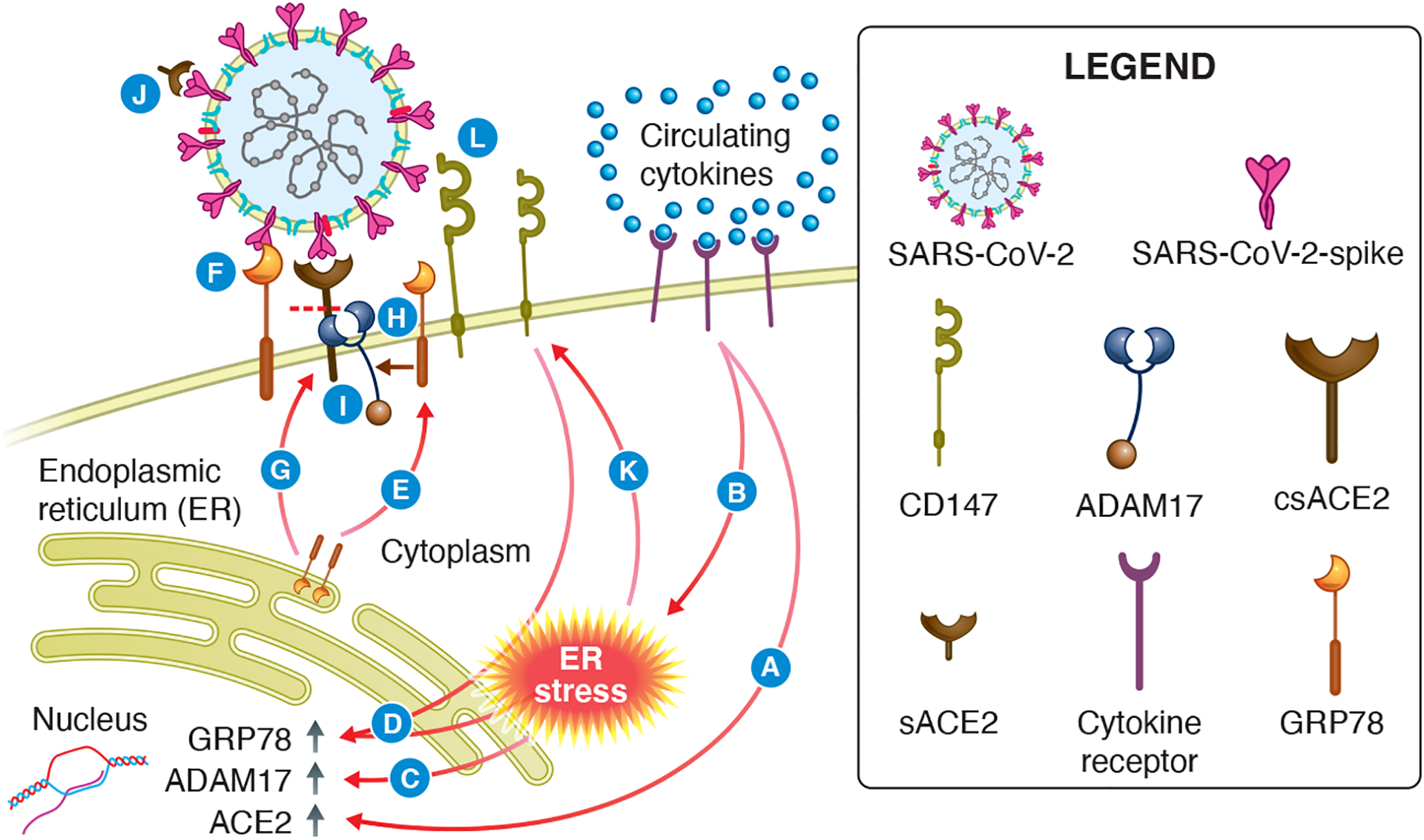
Hypothesized interaction of ACE2, ADAM17, CD147 and GRP78 under conditions of elevated pro-inflammatory cytokines and ER stress. In patients with COVID-19, pancreatic endocrine cells are bathed in cytokines derived from the circulation. This state (A) upregulates ACE2 gene expression and (B) ER stress, which causes (C) an increase in ADAM17 mRNA levels. (D) ER stress also increases GRP78 mRNA levels, mediated by CD147. The subsequent increase in GRP78 protein results in (E) missorting and translocation of GRP78 to the cell surface, where it may (F) act as an alternative receptor or ACE2 cofactor during SARS-CoV-2 entry. (G) GRP78 is also involved in ACE2 trafficking to the plasma membrane. Additionally, GRP78 (H) protects ADAM17 from inhibition, which allows ADAM17 to (I) cleave plasma membrane bound ACE2 at an increased rate, thereby elevating soluble ACE2 levels, which may (J) bind to the spike protein and inhibit SARS-CoV-2 entry. At the same time, (K) ER stress upregulates CD147. The consequent increase in CD147 at the cell surface can (L) increase CD147-mediated SARS-CoV-2 entry.
It is worth noting that SARS-CoV-2 has been shown to infiltrate α cells, therefore more study is needed to understand how this may impact β-cell function, especially since α-to-β cell communication is required for maintaining normal insulin secretion and glycemia. Finally, SARS-CoV-2-induced islet dysfunction likely occurs independent of direct viral entry, perhaps from inflammation and fibrosis in the islet microenvironment; however, the literature would suggest this may be in addition to the direct route of viral invasion.
In sum, systematic investigation of candidate SARS-CoV-2 entry mediators in the endocrine pancreas may inform therapeutic strategies to protect islets from the deleterious effects of COVID-19. Such strategies may need to be continually adapted to keep up with new variants of SARS-CoV-2, whose viral entry mechanisms may differ from previous variants.
Acknowledgements
The authors thank Rebecca Hull-Meichle (VA Puget Sound Health Care System and University of Washington, Seattle, WA, USA) and Sarah Malik (University of Washington, Seattle, WA, USA) for assistance with literature searches, manuscript review, and valuable discussions regarding the content.
Funding
This work was supported by pilot funding to S.Z. from the United States Department of Veterans Affairs, as well as the National Institutes of Health (P30DK017047), and Seattle Institute for Biomedical and Clinical Research.
Footnotes
Declaration of Interest
The authors have no conflicts of interest to declare.
References
- Åkerfeldt MC, Howes J, Chan JY, Stevens VA, Boubenna N, McGuire HM, King C, Biden TJ & Laybutt DR 2008. Cytokine-induced β-Cell death is independent of Endoplasmic Reticulum stress signaling. Diabetes 57 3034–3044. (doi: 10.2337/db07-1802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Aly Z, Xie Y & Bowe B 2021. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594 259–264. (doi: 10.1038/s41586-021-03553-9) [DOI] [PubMed] [Google Scholar]
- Allagnat F, Fukaya M, Nogueira TC, Delaroche D, Welsh N, Marselli L, Marchetti P, Haefliger JA, Eizirik DL & Cardozo AK 2012. C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in β-cells. Cell Death and Differentiation 19 1836–1846. (doi: 10.1038/cdd.2012.67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amati F, Vancheri C, Latini A, Colona VL, Grelli S, D’Apice MR, Balestrieri E, Passarelli C, Minutolo A, Loddo S et al. 2020. Expression profiles of the SARS-CoV-2 host invasion genes in nasopharyngeal and oropharyngeal swabs of COVID-19 patients. Heliyon 6 e05143. (doi: 10.1016/j.heliyon.2020.e05143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Puranik A, Aravamudan M, Venkatakrishnan AJ & Soundararajan V 2020. SARS-CoV-2 strategically mimics proteolytic activation of human ENaC. ELife 9 e58603. (doi: 10.7554/eLife.58603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoe T 2020. Pathological aspects of COVID-19 as a conformational disease and the use of pharmacological chaperones as a potential therapeutic strategy. Frontiers in Pharmacology 11 1095. (doi: 10.3389/fphar.2020.01095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranov MV, Bianchi F & van den Bogaart G 2020. The PIKfyve inhibitor Apilimod: a double-edged sword against COVID-19. Cells 10 30. (doi: 10.3390/cells10010030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian H, Zheng Z-H, Wei D, Wen A, Zhang Z, Lian J-Q, Kang W-Z, Hao C-Q, Wang J, Xie R-H et al. 2021. Safety and efficacy of meplazumab in healthy volunteers and COVID-19 patients: a randomized phase 1 and an exploratory phase 2 trial. Signal Transduction and Targeted Therapy 6 194. (doi: 10.1038/s41392-021-00603-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar GS, Barrow BM, Watson M, Griesbach R, Choung E, Welch A, Ruzsicska B, Raleigh DP & Zraika S 2017. Neprilysin is required for angiotensin-(1–7)’s ability to enhance insulin secretion via its proteolytic activity to generate angiotensin-(1–2). Diabetes 66 2201–2212. (doi: 10.2337/db16-1318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers B, Coppola I, Vints K, Dislich B, Jouvet N, Van Lommel L, Segers C, Gounko NV, Thorrez L, Schuit F et al. 2021. Loss of furin in β cells induces an mTORC1-ATF4 anabolic pathway that leads to β-Cell dysfunction. Diabetes 70 492–503. (doi: 10.2337/db20-0474) [DOI] [PubMed] [Google Scholar]
- Brozzi F, Nardelli TR, Lopes M, Millard I, Barthson J, Igoillo-Esteve M, Grieco FA, Villate O, Oliveira JM, Casimir M et al. 2015. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia 58 2307–2316. (doi: 10.1007/s00125-015-3669-6) [DOI] [PubMed] [Google Scholar]
- Bugliani M, Syed F, Paula FMM, Omar BA, Suleiman M, Mossuto S, Grano F, Cardarelli F, Boggi U, Vistoli F et al. 2018. DPP-4 is expressed in human pancreatic beta cells and its direct inhibition improves beta cell function and survival in type 2 diabetes. Molecular and Cellular Endocrinology 473 186–193. (doi: 10.1016/j.mce.2018.01.019) [DOI] [PubMed] [Google Scholar]
- Cameron K, Rozano L, Falasca M & Mancera RL 2021. Does the SARS-CoV-2 spike protein receptor binding domain interact effectively with the DPP4 (CD26) receptor? A molecular docking study. International Journal of Molecular Sciences 22 7001. (doi: 10.3390/ijms22137001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, van der Meer F, Kallio K, Kaya T, Anastasina M et al. 2020. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science (New York, N.Y.) 370 856–860. (doi: 10.1126/science.abd2985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo AK, Ortis F, Storling J, Feng Y-M, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A & Eizirik DL 2005. Cytokines Downregulate the Sarcoendoplasmic Reticulum Pump Ca2+ ATPase 2b and Deplete Endoplasmic Reticulum Ca2+, Leading to Induction of Endoplasmic Reticulum Stress in Pancreatic β-Cells. Diabetes 54 452 LP–461. (doi: 10.2337/diabetes.54.2.452) [DOI] [PubMed] [Google Scholar]
- Carlos AJ, Ha DP, Yeh D-W, Van Krieken R, Tseng C-C, Zhang P, Gill P, Machida K & Lee AS 2021. The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. The Journal of Biological Chemistry 296 100759. (doi: 10.1016/j.jbc.2021.100759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C-P, Siu K-L, Chin K-T, Yuen K-Y, Zheng B, Jin D-Y, Ching-Ping C, Kam-Leung S, King-Tung C, Kwok-Yung Y et al. 2006. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. Journal of Virology 80 9279–9287. (doi: 10.1128/JVI.00659-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-W, Lee M-S, Lucht A, Chou F-P, Huang W, Havighurst TC, Kim K, Wang J-K, Antalis TM, Johnson MD et al. 2010. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. The American Journal of Pathology 176 2986–2996. (doi: 10.2353/ajpath.2010.090665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Chan C-M, Zhang X, Wang Y, Yuan S, Zhou J, Au-Yeung RK-H, Sze K-H, Yang D, Shuai H et al. 2018. Middle East respiratory syndrome coronavirus and bat coronavirus HKU9 both can utilize GRP78 for attachment onto host cells. The Journal of Biological Chemistry 293 11709–11726. (doi: 10.1074/jbc.RA118.001897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas ECJ, Osterhaus ADME, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, Krauss S, Shortridge KF & Webster RG 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. The Lancet 351 472–477. (doi: 10.1016/S0140-6736(97)11212-0) [DOI] [PubMed] [Google Scholar]
- Clausen TM, Sandoval DR, Spliid CB, Pihl J, Perrett HR, Painter CD, Narayanan A, Majowicz SA, Kwong EM, McVicar RN et al. 2020. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell 183 1043–1057.e15. (doi: 10.1016/j.cell.2020.09.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coate KC, Cha J, Shrestha S, Wang W, Gonçalves LM, Almaça J, Kapp ME, Fasolino M, Morgan A, Dai C et al. 2020. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β Cells. Cell Metabolism 32 1028–1040.e4. (doi: 10.1016/j.cmet.2020.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG & Decroly E 2020. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Research 176 104742. (doi: 10.1016/j.antiviral.2020.104742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly JL, Simonetti B, Klein K, Chen K-E, Williamson MK, Antón-Plágaro C, Shoemark DK, Simón-Gracia L, Bauer M, Hollandi R et al. 2020. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science (New York, N.Y.) 370 861–865. (doi: 10.1126/science.abd3072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana D & Pathak SK 2020. A review of small molecule inhibitors and functional probes of human cathepsin L. Molecules 25 698. (doi: 10.3390/molecules25030698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhounchak S, Popp SK, Brown DJ, Laybutt DR, Biden TJ, Bornstein SR, Parish CR & Simeonovic CJ 2021. Heparan sulfate proteoglycans in beta cells provide a critical link between endoplasmic reticulum stress, oxidative stress and type 2 diabetes. PloS One 16 e0252607. (doi: 10.1371/journal.pone.0252607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Huneidi W, Hamad M & Taneera J 2021. Expression of SARS-CoV-2 receptor “ACE2” in human pancreatic β cells: to be or not to be! Islets 1–9. (doi: 10.1080/19382014.2021.1954458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky AA 2020. SARS-CoV-2 spike-heat shock protein A5 (GRP78) recognition may be related to the immersed human coronaviruses. Frontiers in Pharmacology 11 577467. (doi: 10.3389/fphar.2020.577467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenizia C, Galbiati S, Vanetti C, Vago R, Clerici M, Tacchetti C & Daniele T 2021. SARS-CoV-2 entry: at the crossroads of CD147 and ACE2. Cells 10 1434. (doi: 10.3390/cells10061434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fignani D, Licata G, Brusco N, Nigi L, Grieco GE, Marselli L, Overbergh L, Gysemans C, Colli ML, Marchetti P et al. 2020. SARS-CoV-2 receptor angiotensin I-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Frontiers in Endocrinology 11 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S & Klagsbrun M 2000. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proceedings of the National Academy of Sciences of the United States of America 97 2573–2578. (doi: 10.1073/pnas.040337597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J, Chen L, Yuan Y, Wang K, Wang Y, Qin C, Wu G, Chen R, Zhang Z, Wei D et al. 2021. CD147 antibody specifically and effectively inhibits infection and cytokine storm of SARS-CoV-2 and its variants delta, alpha, beta, and gamma. Signal Transduction and Targeted Therapy 6 347. (doi: 10.1038/s41392-021-00760-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes CP, Fernandes DE, Casimiro F, da Mata GF, Passos MT, Varela P, Mastroianni-Kirsztajn G & Pesquero JB 2020. Cathepsin L in COVID-19: from pharmacological evidences to genetics. Frontiers in Cellular and Infection Microbiology 10 777. (doi: 10.3389/fcimb.2020.589505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass GD & Toole BP 2016. How, with whom and when: an overview of CD147-mediated regulatory networks influencing matrix metalloproteinase activity. Bioscience Reports 36 e00283. (doi: 10.1042/BSR20150256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C & Tang R 2020. Could an endo-lysosomal ion channel be the Achilles heel of SARS-CoV2? Cell Calcium 88 102212. (doi: 10.1016/j.ceca.2020.102212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot S, Delaval P, Brinchault G, Caulet-Maugendre S, Depince A, Lena H, Delatour B, Lagente V & Martin-Chouly C 2006. Increased extracellular matrix metalloproteinase inducer (EMMPRIN) expression in pulmonary fibrosis. Experimental Lung Research 32 81–97. (doi: 10.1080/01902140600710512) [DOI] [PubMed] [Google Scholar]
- Ha DP, Van Krieken R, Carlos AJ & Lee AS 2020. The stress-inducible molecular chaperone GRP78 as potential therapeutic target for coronavirus infection. The Journal of Infection 81 452–482. (doi: 10.1016/j.jinf.2020.06.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S, Yamamoto N, Nakai-Murakami C, Osawa Y, Tokunaga K, Sata T, Yamamoto N, Sasazuki T & Ishizaka Y 2008. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proceedings of the National Academy of Sciences 105 7809–7814. (doi: 10.1073/pnas.0711241105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S, Nagata N, Okamura T, Yamamoto N, Sata T, Yamamoto N, Sasazuki T & Ishizaka Y 2010. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antiviral Research 85 551–555. (doi: 10.1016/j.antiviral.2009.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan NM, Kendrick MA, Druckenbrod NR, Huelsmeyer MK, Warner TF & MacDonald MJ 2010. Genetic association of the neuropilin-1 gene with type 1 diabetes in children: Neuropilin-1 expression in pancreatic islets. Diabetes Research and Clinical Practice 87 e29–e32. (doi: 10.1016/j.diabres.2009.12.016) [DOI] [PubMed] [Google Scholar]
- Helal MA, Shouman S, Abdelwaly A, Elmehrath AO, Essawy M, Sayed SM, Saleh AH & El-Badri N 2020. Molecular basis of the potential interaction of SARS-CoV-2 spike protein to CD147 in COVID-19 associated-lymphopenia. Journal of Biomolecular Structure and Dynamics 1–11. (doi: 10.1080/07391102.2020.1822208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel T, Raich L, Olsson S, Azouz NP, Klingler AM, Hoffmann M, Pöhlmann S, Rothenberg ME & Noé F 2021. Molecular mechanism of inhibiting the SARS-CoV-2 cell entry facilitator TMPRSS2 with camostat and nafamostat. Chemical Science 12 983–992. (doi: 10.1039/D0SC05064D) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O & Pöhlmann S 2014. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. Journal of Virology 88 1293–1307. (doi: 10.1128/JVI.02202-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M & Lindskog C 2020. The protein expression profile of ACE2 in human tissues. Molecular Systems Biology 16 e9610. (doi: 10.15252/msb.20209610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A et al. 2020a. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181 271–280.e8. (doi: 10.1016/j.cell.2020.02.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H & Pöhlmann S 2020b. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Molecular Cell 78 779–784.e5. (doi: 10.1016/j.molcel.2020.04.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Hofmann-Winkler H, Smith JC, Krüger N, Arora P, Sørensen LK, Søgaard OS, Hasselstrøm JB, Winkler M, Hempel T et al. 2021. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine 65. (doi: 10.1016/j.ebiom.2021.103255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yang C, Xu X, Xu W & Liu S 2020. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacologica Sinica 41 1141–1149. (doi: 10.1038/s41401-020-0485-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull RL, Zraika S, Udayasankar J, Aston-Mourney K, Subramanian SL & Kahn SE 2009. Amyloid formation in human IAPP transgenic mouse islets and pancreas, and human pancreas, is not associated with endoplasmic reticulum stress. Diabetologia 52 1102–1111. (doi: 10.1007/s00125-009-1329-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim IM, Abdelmalek DH & Elfiky AA 2019. GRP78: A cell’s response to stress. Life Sciences 226 156–163. (doi: 10.1016/j.lfs.2019.04.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim IM, Abdelmalek DH, Elshahat ME & Elfiky AA 2020. COVID-19 spike-host cell receptor GRP78 binding site prediction. The Journal of Infection 80 554–562. (doi: 10.1016/j.jinf.2020.02.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi R, Kuno T, Komiyama J, Uda K, Miyamoto Y, Taniguchi Y, Abe T, Ishimaru M, Adomi M, Tamiya N et al. 2021. Association between nafamostat mesylate and in-hospital mortality in patients with coronavirus disease 2019: a multicenter observational study. Journal of Clinical Medicine 11 116. (doi: 10.3390/jcm11010116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes JA, Millet JK & Whittaker GR 2020. Proteolytic cleavage of the SARS-CoV-2 spike protein and the role of the novel S1/S2 site. IScience 23 101212. (doi: 10.1016/j.isci.2020.101212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katopodis P, Kerslake R, Davies J, Randeva SH, Chatha K, Hall M, Spandidos AD, Anikin V, Polychronis A, Robertus LJ et al. 2021. COVID-19 and SARS-CoV-2 host cell entry mediators: Expression profiling of TMRSS4 in health and disease. Int J Mol Med 47 64. (doi: 10.3892/ijmm.2021.4897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayo T, Konda Y, Tanaka S, Takata K, Koizumi A & Takeuchi T 1996. Developmental expression of proprotein-processing endoprotease furin in rat pancreatic islets. Endocrinology 137 5126–5134. (doi: 10.1210/endo.137.11.8895387) [DOI] [PubMed] [Google Scholar]
- Ke X, Fei F, Chen Y, Xu L, Zhang Z, Huang Q, Zhang H, Yang H, Chen Z & Xing J 2012. Hypoxia upregulates CD147 through a combined effect of HIF-1α and Sp1 to promote glycolysis and tumor progression in epithelial solid tumors. Carcinogenesis 33 1598–1607. (doi: 10.1093/carcin/bgs196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M, Jeon S, Ryu W-S & Kim S 2021. Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells. Journal of Medical Virology 93 1403–1408. (doi: 10.1002/jmv.26397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köseler A, Sabirli R, Gören T, Türkçüer I & Kurt Ö 2020. Endoplasmic reticulum stress markers in SARS-COV-2 infection and pneumonia: case-control study. In Vivo 34 1645–1650. (doi: 10.21873/invivo.11956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W et al. 2005. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nature Medicine 11 875–879. (doi: 10.1038/nm1267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartseva I, Wu W, Syed F, Van Der Heide V, Jorgensen M, Joseph P, Tang X, Candelario-Jalil E, Yang C, Nick H et al. 2020. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metabolism 32 1041–1051.e6. (doi: 10.1016/j.cmet.2020.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou I, Randeva HS, Spandidos DA & Karteris E 2021. Not only ACE2—the quest for additional host cell mediators of SARS-CoV-2 infection: Neuropilin-1 (NRP1) as a novel SARS-CoV-2 host cell entry mediator implicated in COVID-19. Signal Transduction and Targeted Therapy 6 21. (doi: 10.1038/s41392-020-00460-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laybutt DR, Preston AM, Åkerfeldt MC, Kench JG, Busch AK, Biankin AV & Biden TJ 2007. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50 752–763. (doi: 10.1007/s00125-006-0590-z) [DOI] [PubMed] [Google Scholar]
- Lazartigues E, Qadir MMF & Mauvais-Jarvis F 2020. Endocrine significance of SARS-CoV-2’s reliance on ACE2. Endocrinology 161 bqaa108. (doi: 10.1210/endocr/bqaa108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Kopetz S, Vilar E, Shen JP, Chen K & Maitra A 2020. Relative abundance of SARS-CoV-2 entry genes in the enterocytes of the lower gastrointestinal tract. Genes 11 645. (doi: 10.3390/genes11060645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z & Buck M 2021. Neuropilin-1 assists SARS-CoV-2 infection by stimulating the separation of Spike protein S1 and S2. Biophysical Journal 120 2828–2837. (doi: 10.1016/j.bpj.2021.05.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Z, Yang L, Lian X, Xie Y, Li S, Xin S, Cao P & Lu J 2020. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. IScience 23 101160. (doi: 10.1016/j.isci.2020.101160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Meyerholz DK, Bartlett JA & McCray PBJ 2021. The TMPRSS2 inhibitor nafamostat reduces SARS-CoV-2 pulmonary infection in mouse models of COVID-19. MBio 12 e0097021. (doi: 10.1128/mBio.00970-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lier D, Kox M, Santos K, van der Hoeven H, Pillay J & Pickkers P 2021. Increased blood angiotensin converting enzyme 2 activity in critically ill COVID-19 patients. ERJ Open Research 7 00848–02020. (doi: 10.1183/23120541.00848-2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Long X, Zhang B, Zhang W, Chen X & Zhang Z 2020. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clinical Gastroenterology and Hepatology : The Official Clinical Practice Journal of the American Gastroenterological Association 18 2128–2130.e2. (doi: 10.1016/j.cgh.2020.04.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C-W, Kryvalap Y, Sheu T-J, Chang C-H & Czyzyk J 2019. Cellular proliferation in mouse and human pancreatic islets is regulated by serpin B13 inhibition and downstream targeting of E-cadherin by cathepsin L. Diabetologia 62 822–834. (doi: 10.1007/s00125-019-4834-0) [DOI] [PubMed] [Google Scholar]
- Louagie E, Taylor NA, Flamez D, Roebroek AJM, Bright NA, Meulemans S, Quintens R, Herrera PL, Schuit F, Van de Ven WJM et al. 2008. Role of furin in granular acidification in the endocrine pancreas: identification of the V-ATPase subunit Ac45 as a candidate substrate. Proceedings of the National Academy of Sciences of the United States of America 105 12319–12324. (doi: 10.1073/pnas.0800340105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehr R, Mintern JD, Herman AE, Lennon-Duménil A-M, Mathis D, Benoist C & Ploegh HL 2005. Cathepsin L is essential for onset of autoimmune diabetes in NOD mice. The Journal of Clinical Investigation 115 2934–2943. (doi: 10.1172/JCI25485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL & Cnop M 2007. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 50 2486–2494. (doi: 10.1007/s00125-007-0816-8) [DOI] [PubMed] [Google Scholar]
- Maremanda KP, Sundar IK, Li D & Rahman I 2020. Age-dependent assessment of genes involved in cellular senescence, telomere, and mitochondrial pathways in human lung tissue of smokers, COPD, and IPF: associations with SARS-CoV-2 COVID-19 ACE2-TMPRSS2-Furin-DPP4 Axis. Frontiers in Pharmacology 11 1356. (doi: 10.3389/fphar.2020.584637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marselli L, Piron A, Suleiman M, Colli ML, Yi X, Khamis A, Carrat GR, Rutter GA, Bugliani M, Giusti L et al. 2020. Persistent or transient human β cell dysfunction induced by metabolic stress: specific signatures and shared gene expression with type 2 diabetes. Cell Reports 33 108466. (doi: 10.1016/j.celrep.2020.108466) [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Ujike M, Morikawa S, Tashiro M & Taguchi F 2005. Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proceedings of the National Academy of Sciences of the United States of America 102 12543–12547. (doi: 10.1073/pnas.0503203102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine K, Nagafuchi S, Mori H, Takahashi H & Anzai K 2022. SARS-CoV-2 infection and pancreatic beta-cell failure. Biology 11 22. (doi: 10.3390/biology11010022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Barron E, Vamos E, Thomas S, Dhatariya K, Kar P, Young B, Khunti K & Valabhji J 2021. Temporal trends in emergency admissions for diabetic ketoacidosis in people with diabetes in England before and during the COVID-19 pandemic: a population-based study. The Lancet. Diabetes & Endocrinology 9 671–680. (doi: 10.1016/S2213-8587(21)00208-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, Weil T, Koepke L, Bozzo CP, Read C et al. 2021. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nature Metabolism 3 149–165. (doi: 10.1038/s42255-021-00347-1) [DOI] [PubMed] [Google Scholar]
- Murgolo N, Therien AG, Howell B, Klein D, Koeplinger K, Lieberman LA, Adam GC, Flynn J, McKenna P, Swaminathan G et al. 2021. SARS-CoV-2 tropism, entry, replication, and propagation: Considerations for drug discovery and development. PLOS Pathogens 17 e1009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mycroft-West CJ, Su D, Pagani I, Rudd TR, Elli S, Gandhi NS, Guimond SE, Miller GJ, Meneghetti MCZ, Nader HB et al. 2020. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the spike S1 receptor-binding domain with heparin. Thrombosis and Haemostasis 120 1700–1715. (doi: 10.1055/s-0040-1721319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Zhang Y & Lee AS 2011. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. The Biochemical Journal 434 181–188. (doi: 10.1042/BJ20101569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh Y, Oh I-S, Jeong HE, Filion KB, Yu OHY & Shin J-Y 2021. Association between DPP-4 inhibitors and COVID-19–related outcomes among patients with type 2 diabetes. Diabetes Care 44 e64–e66. (doi: 10.2337/dc20-1824) [DOI] [PubMed] [Google Scholar]
- Omar BA, Liehua L, Yamada Y, Seino Y, Marchetti P & Ahrén B 2014. Dipeptidyl peptidase 4 (DPP-4) is expressed in mouse and human islets and its activity is decreased in human islets from individuals with type 2 diabetes. Diabetologia 57 1876–1883. (doi: 10.1007/s00125-014-3299-4) [DOI] [PubMed] [Google Scholar]
- Onabajo OO, Banday AR, Stanifer ML, Yan W, Obajemu A, Santer DM, Florez-Vargas O, Piontkivska H, Vargas JM, Ring TJ et al. 2020. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nature Genetics 52 1283–1293. (doi: 10.1038/s41588-020-00731-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan P, Desikan R & Dixit NM 2020. Targeting TMPRSS2 and Cathepsin B/L together may be synergistic against SARS-CoV-2 infection. PLoS Computational Biology 16 e1008461. (doi: 10.1371/journal.pcbi.1008461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios Y, Ruiz A, Ramón-Luing LA, Ocaña-Guzman R, Barreto-Rodriguez O, Sánchez-Monciváis A, Tecuatzi-Cadena B, Regalado-García AG, Pineda-Gudiño RD, García-Martínez A et al. 2021. Severe COVID-19 patients show an increase in soluble TNFR1 and ADAM17, with a relationship to mortality. International Journal of Molecular Sciences 22 8423. (doi: 10.3390/ijms22168423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa G, Mallery DL, Albecka A, Welch LG, Cattin-Ortolá J, Luptak J, Paul D, McMahon HT, Goodfellow IG, Carter A et al. 2021. Furin cleavage of SARS-CoV-2 Spike promotes but is not essential for infection and cell-cell fusion. PLOS Pathogens 17 e1009246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock TP, Goldhill DH, Zhou J, Baillon L, Frise R, Swann OC, Kugathasan R, Penn R, Brown JC, Sanchez-David RY et al. 2021. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nature Microbiology 6 899–909. (doi: 10.1038/s41564-021-00908-w) [DOI] [PubMed] [Google Scholar]
- Pellet-Many C, Frankel P, Jia H & Zachary I 2008. Neuropilins: structure, function and role in disease. Biochemical Journal 411 211–226. (doi: 10.1042/BJ20071639) [DOI] [PubMed] [Google Scholar]
- Pirot P, Eizirik DL & Cardozo AK 2006. Interferon-γ potentiates endoplasmic reticulum stress-induced death by reducing pancreatic beta cell defence mechanisms. Diabetologia 49 1229. (doi: 10.1007/s00125-006-0214-7) [DOI] [PubMed] [Google Scholar]
- Qadir MMF, Bhondeley M, Beatty W, Gaupp DD, Doyle-Meyers LA, Fischer T, Bandyopadhyay I, Blair RV, Bohm R, Rappaport J et al. 2021. SARS-CoV-2 infection of the pancreas promotes thrombofibrosis and is associated with new-onset diabetes. JCI Insight 6 e151551. (doi: 10.1172/jci.insight.151551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TM, Gaughan EE, Bruce A, Antonelli J, O’Connor R, Li F, McNamara S, Koch O, MacKintosh C, Dockrell D et al. 2022. Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: Phase 1b/2a experimental study to investigate safety, Pharmacokinetics and Pharmacodynamics. EBioMedicine 76 103856. (doi: 10.1016/j.ebiom.2022.103856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragotte RJ, Pulido D, Donnellan FR, Hill ML, Gorini G, Davies H, Brun J, McHugh K, King LDW, Skinner K et al. 2021. Human basigin (CD147) does not directly interact with SARS-CoV-2 spike glycoprotein. MSphere 6 e0064721–e0064721. (doi: 10.1128/mSphere.00647-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Fernandes L, Lazari LC, da Silva JM, de Morais Gomes V, Machado RRG, dos Santos AF, Araujo DB, Coutinho JVP, Arini GS, Angeli CB et al. 2021. SARS-CoV-2 activates ER stress and unfolded protein response. BioRxiv 2021.06.21.449284. (doi: 10.1101/2021.06.21.449284) [DOI] [Google Scholar]
- Rzymski T, Petry A, Kračun D, Rieß F, Pike L, Harris AL & Görlach A 2012. The unfolded protein response controls induction and activation of ADAM17/TACE by severe hypoxia and ER stress. Oncogene 31 3621–3634. (doi: 10.1038/onc.2011.522) [DOI] [PubMed] [Google Scholar]
- Sabirli R, Koseler A, Goren T, Turkcuer I & Kurt O 2021. High GRP78 levels in Covid-19 infection: A case-control study. Life Sciences 265 118781. (doi: 10.1016/j.lfs.2020.118781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K et al. 2019. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Research and Clinical Practice 157 107843. (doi: 10.1016/j.diabres.2019.107843) [DOI] [PubMed] [Google Scholar]
- Sawada Y, Kameya T, Aizama T, Izumi T & Takeuchi T 2000. Proprotein-processing endoprotease furin and its substrate parathyroid hormone-related protein are coexpressed in insulinoma cells. Endocrine Pathology 11 31–39. (doi: 10.1385/ep:11:1:31) [DOI] [PubMed] [Google Scholar]
- Schäfer M, Granato DC, Krossa S, Bartels A-K, Yokoo S, Düsterhöft S, Koudelka T, Scheidig AJ, Tholey A, Paes Leme AF et al. 2017. GRP78 protects a disintegrin and metalloprotease 17 against protein-disulfide isomerase A6 catalyzed inactivation. FEBS Letters 591 3567–3587. (doi: 10.1002/1873-3468.12858) [DOI] [PubMed] [Google Scholar]
- Schreiber B, Patel A & Verma A 2021. Shedding light on COVID-19: ADAM17 the missing link? American Journal of Therapeutics 28. [DOI] [PubMed] [Google Scholar]
- Segerstolpe Å, Palasantza A, Eliasson P, Andersson E-M, Andréasson A-C, Sun X, Picelli S, Sabirsh A, Clausen M, Bjursell MK et al. 2016. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metabolism 24 593–607. (doi: 10.1016/j.cmet.2016.08.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A & Li F 2020. Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences of the United States of America 117 11727–11734. (doi: 10.1073/pnas.2003138117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J, Sciaky N, Lee J, Bosshart H, Angeletti RH & Bonifacino JS 1997. Localization of endogenous furin in cultured cell lines. The Journal of Histochemistry and Cytochemistry : Official Journal of the Histochemistry Society 45 3–12. (doi: 10.1177/002215549704500102) [DOI] [PubMed] [Google Scholar]
- Shilts J, Crozier TWM, Greenwood EJD, Lehner PJ & Wright GJ 2021. No evidence for basigin/CD147 as a direct SARS-CoV-2 spike binding receptor. Scientific Reports 11 413. (doi: 10.1038/s41598-020-80464-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenblock C, Richter S, Berger I, Barovic M, Schmid J, Schubert U, Jarzebska N, von Mässenhausen A, Linkermann A, Schürmann A et al. 2021. Viral infiltration of pancreatic islets in patients with COVID-19. Nature Communications 12 3534. (doi: 10.1038/s41467-021-23886-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I, Noguchi N, Nata K, Yamada S, Kaneiwa T, Mizumoto S, Ikeda T, Sugihara K, Asano M, Yoshikawa T et al. 2009. Important role of heparan sulfate in postnatal islet growth and insulin secretion. Biochemical and Biophysical Research Communications 383 113–118. (doi: 10.1016/j.bbrc.2009.03.140) [DOI] [PubMed] [Google Scholar]
- Taneera J, El-Huneidi W, Hamad M, Mohammed AK, Elaraby E & Hachim MY 2020. Expression profile of SARS-CoV-2 host receptors in human pancreatic islets revealed upregulation of ACE2 in diabetic donors. Biology 9 215. (doi: 10.3390/biology9080215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Guo Y-S, Zhang Y, Yu X-L, Li L, Huang W, Li Y, Chen B, Jiang J-L & Chen Z-N 2012. CD147 induces UPR to inhibit apoptosis and chemosensitivity by increasing the transcription of Bip in hepatocellular carcinoma. Cell Death & Differentiation 19 1779–1790. (doi: 10.1038/cdd.2012.60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Yang M, Duan Z, Liao Z, Liu L, Cheng R, Fang M, Wang G, Liu H, Xu J et al. 2020. Transferrin receptor is another receptor for SARS-CoV-2 entry. BioRxiv 2020.10.23.350348. (doi: 10.1101/2020.10.23.350348) [DOI] [Google Scholar]
- Tang X, Uhl S, Zhang T, Xue D, Li B, Vandana JJ, Acklin JA, Bonnycastle LL, Narisu N, Erdos MR et al. 2021a. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metabolism 33 1577–1591.e7. (doi: 10.1016/j.cmet.2021.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T, Jaimes JA, Bidon MK, Straus MR, Daniel S & Whittaker GR 2021b. Proteolytic activation of SARS-CoV-2 Spike at the S1/S2 Boundary: potential role of proteases beyond furin. ACS Infectious Diseases 7 264–272. (doi: 10.1021/acsinfecdis.0c00701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenti A, Arvin A, Corey L, Corti D, Diamond MS, García-Sastre A, Garry RF, Holmes EC, Pang PS & Virgin HW 2021. After the pandemic: perspectives on the future trajectory of COVID-19. Nature 596 495–504. (doi: 10.1038/s41586-021-03792-w) [DOI] [PubMed] [Google Scholar]
- Thomas G 2002. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nature Reviews. Molecular Cell Biology 3 753–766. (doi: 10.1038/nrm934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A et al. 2015. Proteomics. Tissue-based map of the human proteome. Science (New York, N.Y.) 347 1260419. (doi: 10.1126/science.1260419) [DOI] [PubMed] [Google Scholar]
- Ulrich H & Pillat MM 2020. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Reviews and Reports 16 434–440. (doi: 10.1007/s12015-020-09976-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankadari N & Wilce JA 2020. Emerging COVID-19 coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerging Microbes & Infections 9 601–604. (doi: 10.1080/22221751.2020.1739565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellanki P & Umpierrez GE 2017. Diabetic ketoacidosis: a common debut of diabetes among African Americans with type 2 diabetes. Endocrine Practice 23 971–978. (doi: 10.4158/EP161679.RA) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wander PL, Lowy E, Beste LA, Tulloch-Palomino L, Korpak A, Peterson AC, Young BA & Boyko EJ 2021. Risk factors for adverse outcomes among 35 879 veterans with and without diabetes after diagnosis with COVID-19. BMJ Open Diabetes Research & Care 9 e002252. (doi: 10.1136/bmjdrc-2021-002252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wander PL, Lowy E, Beste LA, Tulloch-Palomino L, Korpak A, Peterson AC, Kahn SE & Boyko EJ 2022. The incidence of diabetes among 2,777,768 veterans with and without recent SARS-CoV-2 infection. Diabetes Care 45 782–788. (doi: 10.2337/dc21-1686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang P, Peng J-L, Wu S, Zhao X-P, Li L & Shen G-X 2009. The altered expression of glucose-regulated proteins 78 in different phase of streptozotocin-affected pancreatic beta-cells. Cell Stress & Chaperones 14 43–48. (doi: 10.1007/s12192-008-0053-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Chen W, Zhang Z, Deng Y, Lian J-Q, Du P, Wei D, Zhang Y, Sun X-X, Gong L et al. 2020. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduction and Targeted Therapy 5 283. (doi: 10.1038/s41392-020-00426-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Wan L, Yan Q, Wang X, Zhang J, Yang X, Zhang Y, Fan C, Li D, Deng Y et al. 2020. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nature Metabolism 2 1391–1400. (doi: 10.1038/s42255-020-00324-0) [DOI] [PubMed] [Google Scholar]
- Wruck W & Adjaye J 2020. SARS-CoV-2 receptor ACE2 is co-expressed with genes related to transmembrane serine proteases, viral entry, immunity and cellular stress. Scientific Reports 10 21415. (doi: 10.1038/s41598-020-78402-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-T, Lidsky PV, Xiao Y, Lee IT, Cheng R, Nakayama T, Jiang S, Demeter J, Bevacqua RJ, Chang CA et al. 2021. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metabolism 33 1565–1576.e5. (doi: 10.1016/j.cmet.2021.05.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi CR, Di Fazio A, Nadvi NA, Patel K, Xiang MS, Zhang HE, Deshpande C, Low JK, Wang XT, Chen Y et al. 2020. A novel purification procedure for active recombinant human DPP4 and the inability of DPP4 to bind SARS-CoV-2. Molecules 25 5392. (doi: 10.3390/molecules25225392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y & Al-Aly Z 2022. Risks and burdens of incident diabetes in long COVID: a cohort study. The Lancet. Diabetes & Endocrinology. (doi: 10.1016/S2213-8587(22)00044-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadati T, Houben T, Bitorina A & Shiri-Sverdlov R 2020. The ins and outs of cathepsins: physiological function and role in disease management. Cells 9 1679. (doi: 10.3390/cells9071679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-K, Lin S-S, Ji X-J & Guo L-M 2010. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetologica 47 193–199. (doi: 10.1007/s00592-009-0109-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F et al. 2020. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell 27 125–136.e7. (doi: 10.1016/j.stem.2020.06.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cai Z & Zhang J 2021. DPP-4 inhibitors may improve the mortality of coronavirus disease 2019: A meta-analysis. PLOS ONE 16 e0251916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung ML, Teng JLL, Jia L, Zhang C, Huang C, Cai J-P, Zhou R, Chan K-H, Zhao H, Zhu L et al. 2021. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell 184 2212–2228.e12. (doi: 10.1016/j.cell.2021.02.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang R, Castro MFG, McCune BT, Zeng Q, Rothlauf PW, Sonnek NM, Liu Z, Brulois KF, Wang X, Greenberg HB et al. 2020. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Science Immunology 5 eabc3582. (doi: 10.1126/sciimmunol.abc3582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Chen CZ, Swaroop M, Xu M, Wang L, Lee J, Wang AQ, Pradhan M, Hagen N, Chen L et al. 2020. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discovery 6 80. (doi: 10.1038/s41421-020-00222-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Wilson MC, Schuit F, Halestrap AP & Rutter GA 2001. Expression and distribution of lactate/monocarboxylate transporter isoforms in pancreatic islets and the exocrine pancreas. Diabetes 50 361–366. (doi: 10.2337/diabetes.50.2.361) [DOI] [PubMed] [Google Scholar]
- Zhao M-M, Yang W-L, Yang F-Y, Zhang L, Huang W-J, Hou W, Fan C-F, Jin R-H, Feng Y-M, Wang Y-C et al. 2021. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduction and Targeted Therapy 6 134. (doi: 10.1038/s41392-021-00558-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Feng F, Hu G, Wang Y, Yu Y, Zhu Y, Xu W, Cai X, Sun Z, Han W et al. 2021. A genome-wide CRISPR screen identifies host factors that regulate SARS-CoV-2 entry. Nature Communications 12 961. (doi: 10.1038/s41467-021-21213-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuravel SV, Khmelnitskiy OK, Burlaka OO, Gritsan AI, Goloshchekin BM, Kim S & Hong KY 2021. Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: a randomised Phase II clinical trial. EClinicalMedicine 41 101169. (doi: 10.1016/j.eclinm.2021.101169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowski AF, Popp SK, Freeman C, Parish CR & Simeonovic CJ 2012. Heparan sulfate and heparanase play key roles in mouse β cell survival and autoimmune diabetes. The Journal of Clinical Investigation 122 132–141. (doi: 10.1172/JCI46177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipeto D, Palmeira J da F, Argañaraz GA & Argañaraz ER 2020. ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID-19. Frontiers in Immunology 11 2642. (doi: 10.3389/fimmu.2020.576745) [DOI] [PMC free article] [PubMed] [Google Scholar]


