Abstract
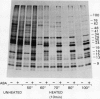
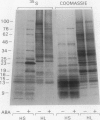
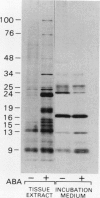
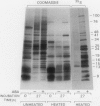
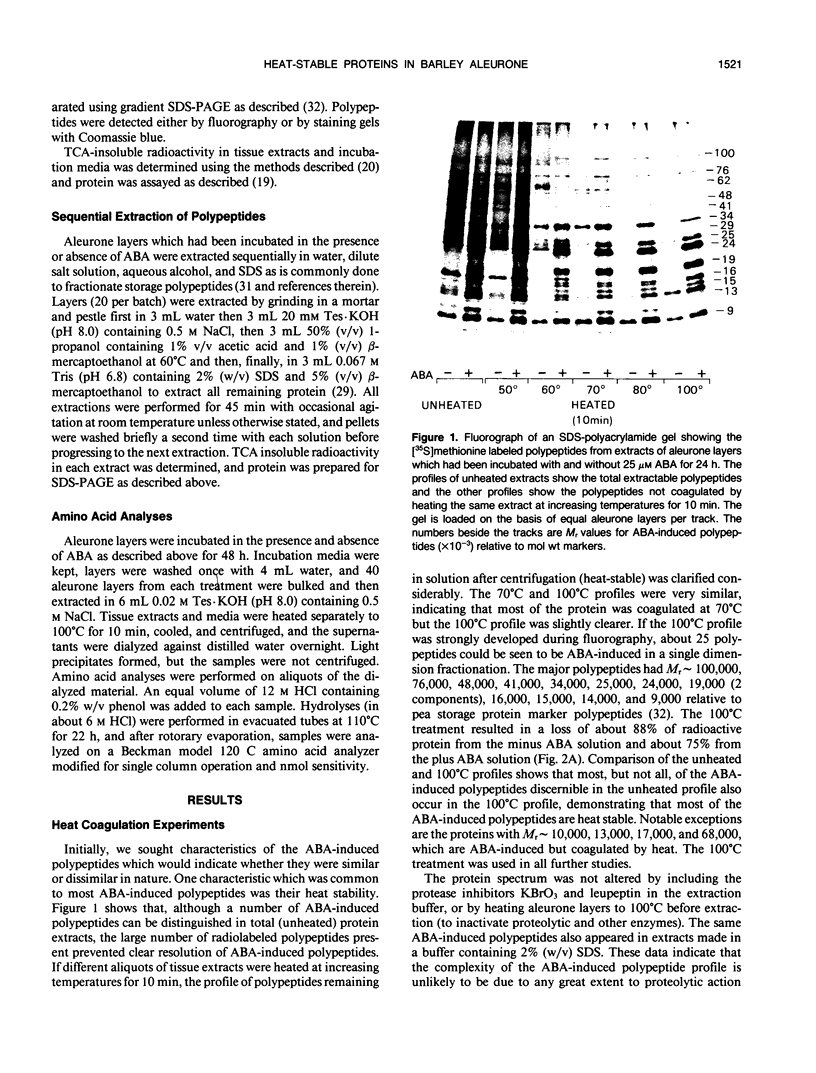
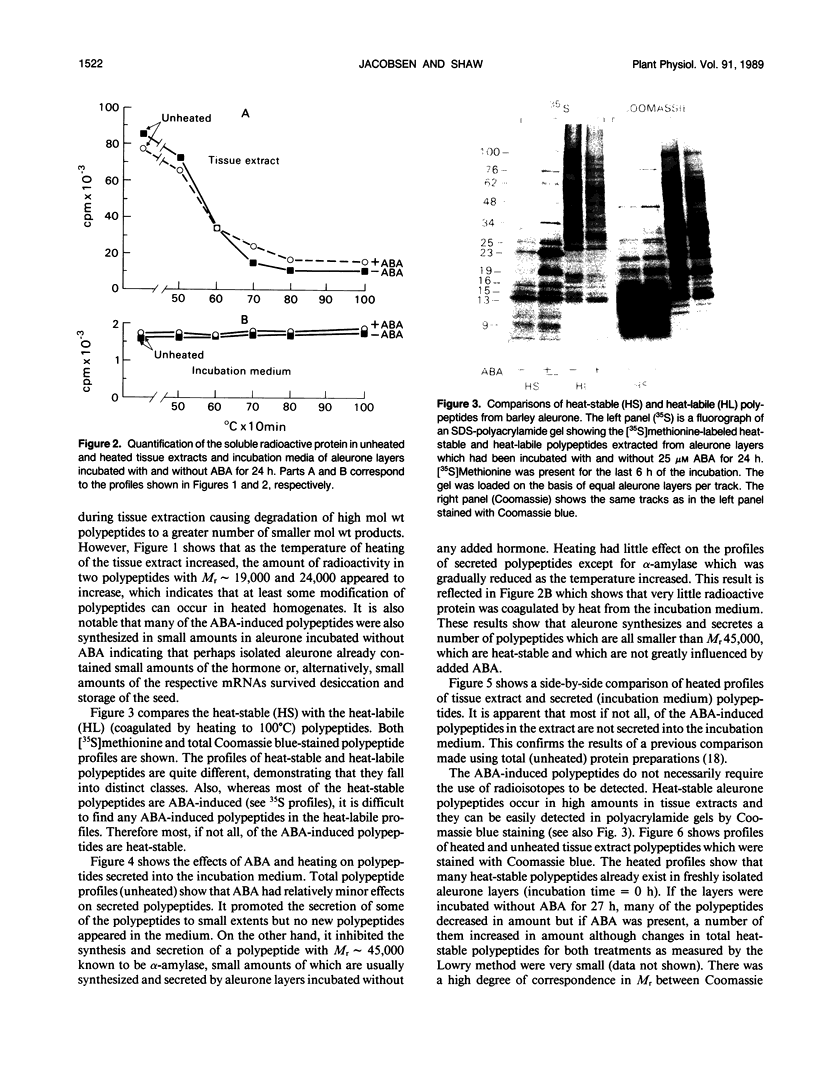
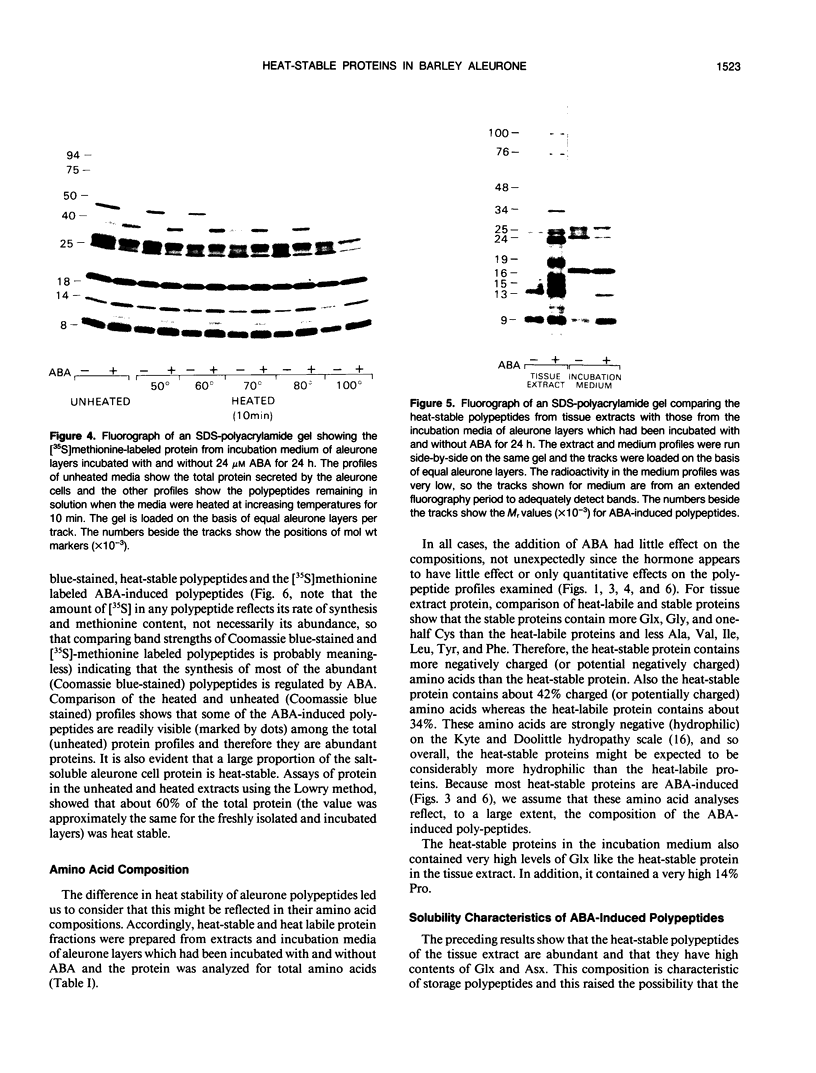
[35S]Methionine labeling experiments showed that abscisic acid (ABA) induced the synthesis of at least 25 polypeptides in mature barley (Hordeum vulgare) aleurone cells. The polypeptides were not secreted. Whereas most of the proteins extracted from aleurone cells were coagulated by heating to 100°C for 10 minutes, most of the ABA-induced polypeptides remained in solution (heat-stable). ABA had little effect on the spectrum of polypeptides that were synthesized and secreted by aleurone cells, and most of these secreted polypeptides were also heatstable. Coomassie blue staining of sodium dodecyl sulfate polyacrylamide gels indicated that ABA-induced polypeptides already occurred in high amounts in mature aleurone layers having accumulated during grain development. About 60% of the total protein extracted from mature aleurone was heat stable. Amino acid analyses of total preparations of heat-stable and heat-labile proteins showed that, compared to heat-labile proteins, heat-stable intracellular proteins were characterized by higher glutamic acid/glutamine (Glx) and glycine levels and lower levels of neutral amino acids. Secreted heat-stable proteins were rich in Glx and proline. The possibilities that the accumulation of the heat-stable polypeptides during grain development is controlled by ABA and that the function of these polypeptides is related to their abundance and extraordinary heat stability are considered.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amelunxen R. E., Murdock A. L. Mechanisms of thermophily. CRC Crit Rev Microbiol. 1978;6(4):343–393. doi: 10.3109/10408417809090626. [DOI] [PubMed] [Google Scholar]
- Anfinsen C. B., Scheraga H. A. Experimental and theoretical aspects of protein folding. Adv Protein Chem. 1975;29:205–300. doi: 10.1016/s0065-3233(08)60413-1. [DOI] [PubMed] [Google Scholar]
- Bray E. A., Beachy R. N. Regulation by ABA of beta-Conglycinin Expression in Cultured Developing Soybean Cotyledons. Plant Physiol. 1985 Nov;79(3):746–750. doi: 10.1104/pp.79.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galau G. A., Bijaisoradat N., Hughes D. W. Accumulation kinetics of cotton late embryogenesis-abundant mRNAs and storage protein mRNAs: coordinate regulation during embryogenesis and the role of abscisic acid. Dev Biol. 1987 Sep;123(1):198–212. doi: 10.1016/0012-1606(87)90442-8. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin L. S., Ho T. H. Mode of action of abscisic Acid in barley aleurone layers : induction of new proteins by abscisic Acid. Plant Physiol. 1986 Sep;82(1):289–297. doi: 10.1104/pp.82.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M., Yasumura S., Aiba S. Cumulative effect of intragenic amino-acid replacements on the thermostability of a protein. 1986 Sep 25-Oct 1Nature. 323(6086):356–358. doi: 10.1038/323356a0. [DOI] [PubMed] [Google Scholar]
- Minton K. W., Karmin P., Hahn G. M., Minton A. P. Nonspecific stabilization of stress-susceptible proteins by stress-resistant proteins: a model for the biological role of heat shock proteins. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7107–7111. doi: 10.1073/pnas.79.23.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozer T. J. Control of protein synthesis in barley aleurone layers by the plant hormones gibberellic acid and abscisic acid. Cell. 1980 Jun;20(2):479–485. doi: 10.1016/0092-8674(80)90634-0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Electrostatic effects in proteins. Science. 1978 Sep 29;201(4362):1187–1191. doi: 10.1126/science.694508. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Raidt H. Stereochemical basis of heat stability in bacterial ferredoxins and in haemoglobin A2. Nature. 1975 May 15;255(5505):256–259. doi: 10.1038/255256a0. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Spencer D., Higgins T. J., Button S. C., Davey R. A. Pulse-labeling Studies on Protein Synthesis in Developing Pea Seeds and Evidence of a Precursor Form of Legumin Small Subunit. Plant Physiol. 1980 Sep;66(3):510–515. doi: 10.1104/pp.66.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J. M. Disulphide bridges in globular proteins. J Mol Biol. 1981 Sep 15;151(2):261–287. doi: 10.1016/0022-2836(81)90515-5. [DOI] [PubMed] [Google Scholar]
- Tomazic S. J., Klibanov A. M. Why is one Bacillus alpha-amylase more resistant against irreversible thermoinactivation than another? J Biol Chem. 1988 Mar 5;263(7):3092–3096. [PubMed] [Google Scholar]
- Triplett B. A., Quatrano R. S. Timing, localization, and control of wheat germ agglutinin synthesis in developing wheat embryos. Dev Biol. 1982 Jun;91(2):491–496. doi: 10.1016/0012-1606(82)90057-4. [DOI] [PubMed] [Google Scholar]
- Uknes S. J., Ho T. H. Mode of action of abscisic Acid in barley aleurone layers : abscisic Acid induces its own conversion to phaseic Acid. Plant Physiol. 1984 Aug;75(4):1126–1132. doi: 10.1104/pp.75.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]