Abstract
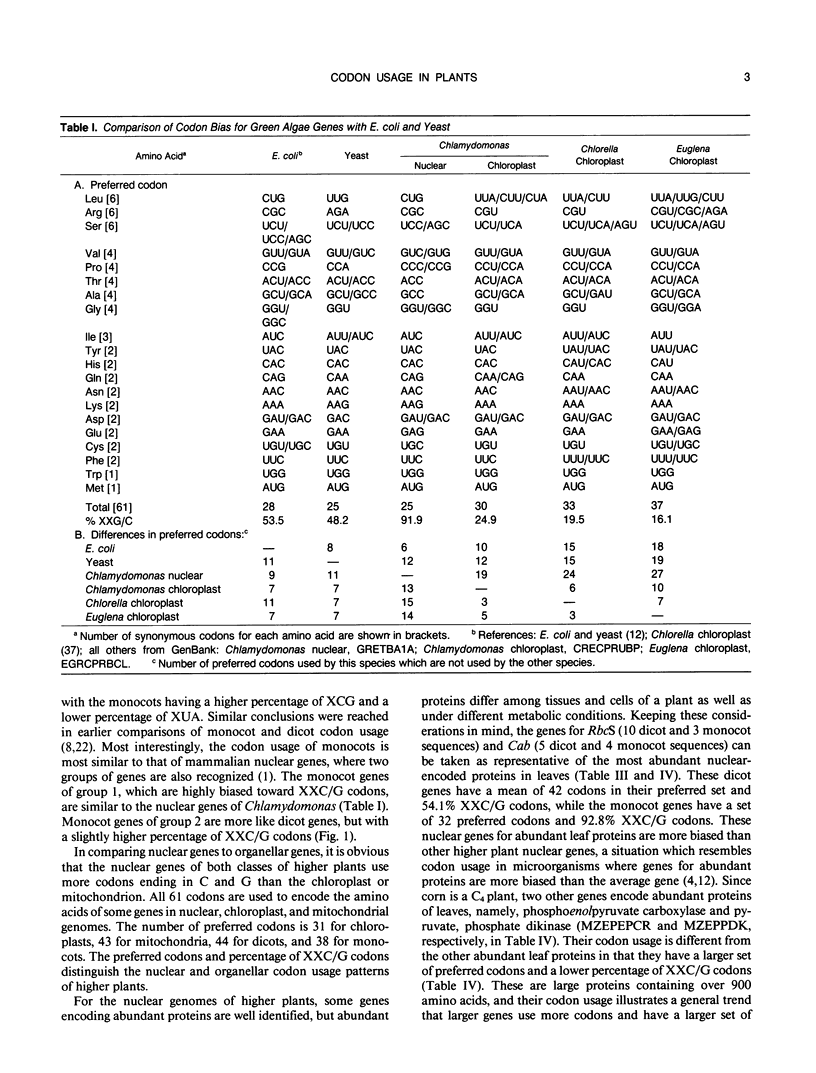
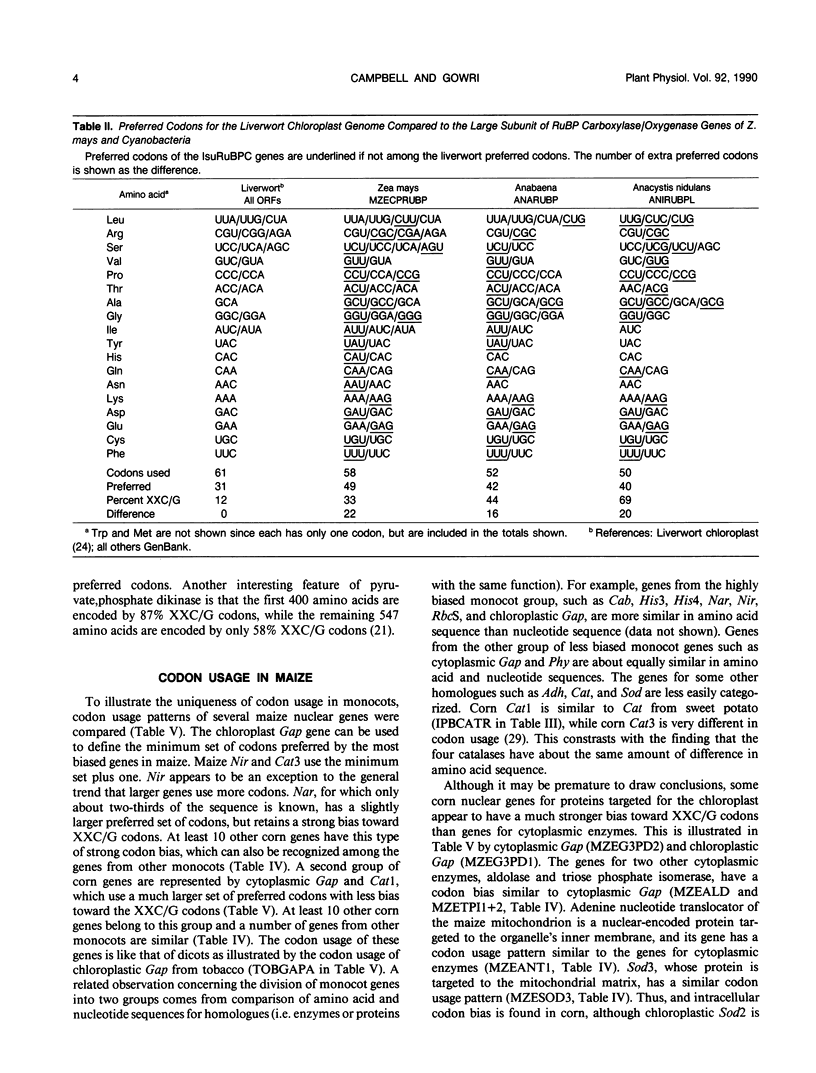
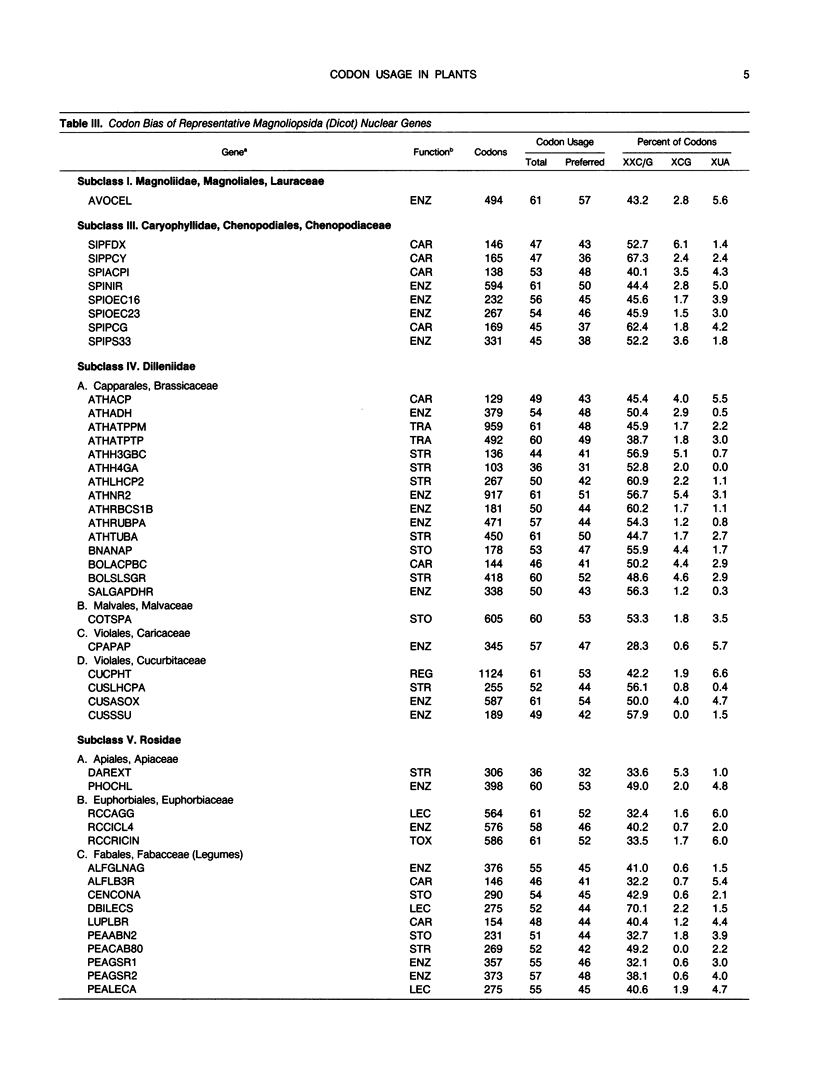
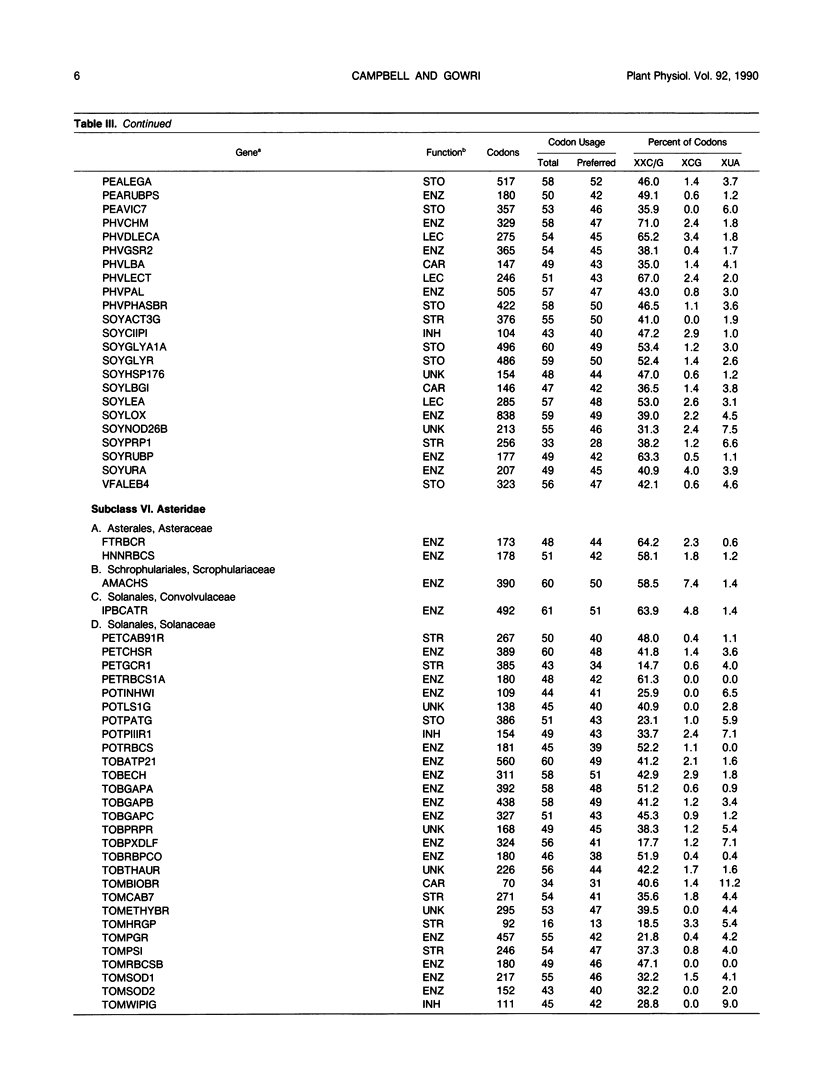
Codon usage is the selective and nonrandom use of synonymous codons by an organism to encode the amino acids in the genes for its proteins. During the last few years, a large number of plant genes have been cloned and sequenced, which now permits a meaningful comparison of codon usage in higher plants, algae, and cyanobacteria. For the nuclear and organellar genes of these organisms, a small set of preferred codons are used for encoding proteins. Codon usage is different for each genome type with the variation mainly occurring in choices between codons ending in cytidine (C) or guanosine (G) versus those ending in adenosine (A) or uridine (U). For organellar genomes, chloroplastic and mitochrondrial proteins are encoded mainly with codons ending in A or U. In most cyanobacteria and the nuclei of green algae, proteins are encoded preferentially with codons ending in C or G. Although only a few nuclear genes of higher plants have been sequenced, a clear distinction between Magnoliopsida (dicot) and Liliopsida (monocot) codon usage is evident. Dicot genes use a set of 44 preferred codons with a slight preference for codons ending in A or U. Monocot codon usage is more restricted with an average of 38 codons preferred, which are predominantly those ending in C or G. But two classes of genes can be recognized in monocots. One set of monocot genes uses codons similar to those in dicots, while the other genes are highly biased toward codons ending in C or G with a pattern similar to nuclear genes of green algae. Codon usage is discussed in relation to evolution of plants and prospects for intergenic transfer of particular genes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aota S., Ikemura T. Diversity in G + C content at the third position of codons in vertebrate genes and its cause. Nucleic Acids Res. 1986 Aug 26;14(16):6345–6355. doi: 10.1093/nar/14.16.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back E., Burkhart W., Moyer M., Privalle L., Rothstein S. Isolation of cDNA clones coding for spinach nitrite reductase: complete sequence and nitrate induction. Mol Gen Genet. 1988 Apr;212(1):20–26. doi: 10.1007/BF00322440. [DOI] [PubMed] [Google Scholar]
- Belanger F. C., Kriz A. L. Molecular characterization of the major maize embryo globulin encoded by the glb1 gene. Plant Physiol. 1989 Oct;91(2):636–643. doi: 10.1104/pp.91.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Bethards L. A., Skadsen R. W., Scandalios J. G. Isolation and characterization of a cDNA clone for the Cat2 gene in maize and its homology with other catalases. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6830–6834. doi: 10.1073/pnas.84.19.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E., Gelbart T., Han J. H., Koziol J. A., Beutler B. Evolution of the genome and the genetic code: selection at the dinucleotide level by methylation and polyribonucleotide cleavage. Proc Natl Acad Sci U S A. 1989 Jan;86(1):192–196. doi: 10.1073/pnas.86.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Brinkmann H., Martinez P., Quigley F., Martin W., Cerff R. Endosymbiotic origin and codon bias of the nuclear gene for chloroplast glyceraldehyde-3-phosphate dehydrogenase from maize. J Mol Evol. 1987;26(4):320–328. doi: 10.1007/BF02101150. [DOI] [PubMed] [Google Scholar]
- Cannon R. E., White J. A., Scandalios J. G. Cloning of cDNA for maize superoxide dismutase 2 (SOD2). Proc Natl Acad Sci U S A. 1987 Jan;84(1):179–183. doi: 10.1073/pnas.84.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford N. M., Smith M., Bellissimo D., Davis R. W. Sequence and nitrate regulation of the Arabidopsis thaliana mRNA encoding nitrate reductase, a metalloflavoprotein with three functional domains. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5006–5010. doi: 10.1073/pnas.85.14.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowri G., Campbell W. H. cDNA Clones for Corn Leaf NADH:Nitrate Reductase and Chloroplast NAD(P):Glyceraldehyde-3-Phosphate Dehydrogenase : Characterization of the Clones and Analysis of the Expression of the Genes in Leaves as Influenced by Nitrate in the Light and Dark. Plant Physiol. 1989 Jul;90(3):792–798. doi: 10.1104/pp.90.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Surowy T. K., Sussman M. R. Molecular cloning and sequence of cDNA encoding the plasma membrane proton pump (H+-ATPase) of Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1234–1238. doi: 10.1073/pnas.86.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahners K., Kramer V., Back E., Privalle L., Rothstein S. Molecular cloning of complementary DNA encoding maize nitrite reductase: molecular analysis and nitrate induction. Plant Physiol. 1988 Nov;88(3):741–746. doi: 10.1104/pp.88.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S., Bogorad L. Nucleotide sequences of two genes encoding the light harvesting chlorophyll a/b binding protein of rice. Nucleic Acids Res. 1989 Mar 25;17(6):2357–2358. doi: 10.1093/nar/17.6.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolson M. F., Ouellette B. F., Filion M., Poole R. J. cDNA sequence and homologies of the "57-kDa" nucleotide-binding subunit of the vacuolar ATPase from Arabidopsis. J Biol Chem. 1988 Dec 5;263(34):17987–17994. [PubMed] [Google Scholar]
- Matsuoka M., Ozeki Y., Yamamoto N., Hirano H., Kano-Murakami Y., Tanaka Y. Primary structure of maize pyruvate, orthophosphate dikinase as deduced from cDNA sequence. J Biol Chem. 1988 Aug 15;263(23):11080–11083. [PubMed] [Google Scholar]
- Murray E. E., Lotzer J., Eberle M. Codon usage in plant genes. Nucleic Acids Res. 1989 Jan 25;17(2):477–498. doi: 10.1093/nar/17.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa J., Okada N., Shinmyo A., Takano M. Primary structure of cucumber (Cucumis sativus) ascorbate oxidase deduced from cDNA sequence: homology with blue copper proteins and tissue-specific expression. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1239–1243. doi: 10.1073/pnas.86.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Pfitzinger H., Guillemaut P., Weil J. H., Pillay D. T. Adjustment of the tRNA population to the codon usage in chloroplasts. Nucleic Acids Res. 1987 Feb 25;15(4):1377–1386. doi: 10.1093/nar/15.4.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post-Beittenmiller M. A., Hlousek-Radojcić A., Ohlrogge J. B. DNA sequence of a genomic clone encoding an Arabidopsis acyl carrier protein (ACP). Nucleic Acids Res. 1989 Feb 25;17(4):1777–1777. doi: 10.1093/nar/17.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley F., Martin W. F., Cerff R. Intron conservation across the prokaryote-eukaryote boundary: structure of the nuclear gene for chloroplast glyceraldehyde-3-phosphate dehydrogenase from maize. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2672–2676. doi: 10.1073/pnas.85.8.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh M. G., Wadsworth G. J., Scandalios J. G. Characterization of catalase transcripts and their differential expression in maize. Biochim Biophys Acta. 1988 Nov 10;951(1):104–116. doi: 10.1016/0167-4781(88)90030-9. [DOI] [PubMed] [Google Scholar]
- Springer B. A., Sligar S. G. High-level expression of sperm whale myoglobin in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8961–8965. doi: 10.1073/pnas.84.24.8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey S. V., Walker E. L., Coruzzi G. M. Glutamine synthetase genes of pea encode distinct polypeptides which are differentially expressed in leaves, roots and nodules. EMBO J. 1987 Jan;6(1):1–9. doi: 10.1002/j.1460-2075.1987.tb04710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke J. M., Ogren W. L. Structure of an Arabidopsis thaliana cDNA encoding rubisco activase. Nucleic Acids Res. 1989 Apr 11;17(7):2871–2871. doi: 10.1093/nar/17.7.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. A., Scandalios J. G. Isolation and characterization of a cDNA for mitochondrial manganese superoxide dismutase (SOD-3) of maize and its relation to other manganese superoxide dismutases. Biochim Biophys Acta. 1988 Nov 10;951(1):61–70. doi: 10.1016/0167-4781(88)90025-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Kano-Murakami Y., Matsuoka M., Ohashi Y., Tanaka Y. Nucleotide sequence of a full length cDNA clone of ribulose bisphosphate carboxylase small subunit gene from green dark-grown pine (Pinus tunbergii) seedling. Nucleic Acids Res. 1988 Dec 23;16(24):11830–11830. doi: 10.1093/nar/16.24.11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Matsuoka M., Kano-Murakami Y., Tanaka Y., Ohashi Y. Nucleotide sequence of a full length cDNA clone of light harvesting chlorophyll a/b binding protein gene from green dark-grown pine (Pinus tunbergii) seedling. Nucleic Acids Res. 1988 Dec 23;16(24):11829–11829. doi: 10.1093/nar/16.24.11829. [DOI] [PMC free article] [PubMed] [Google Scholar]


