Abstract
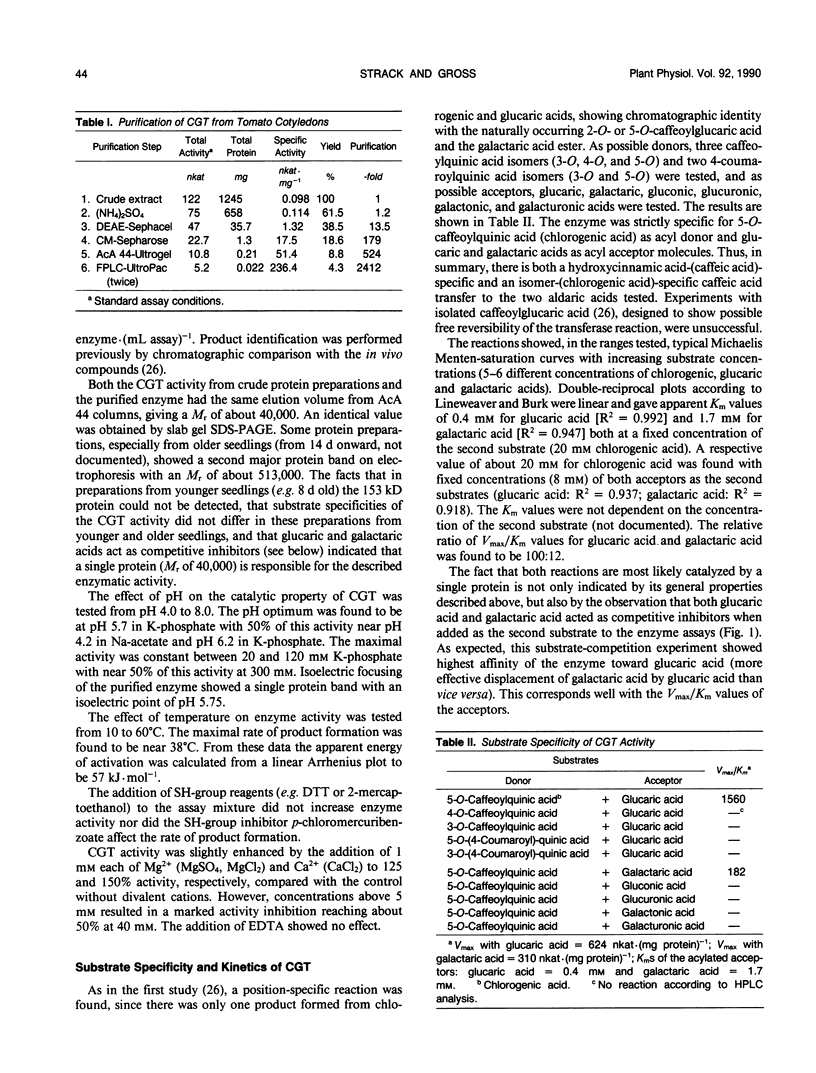
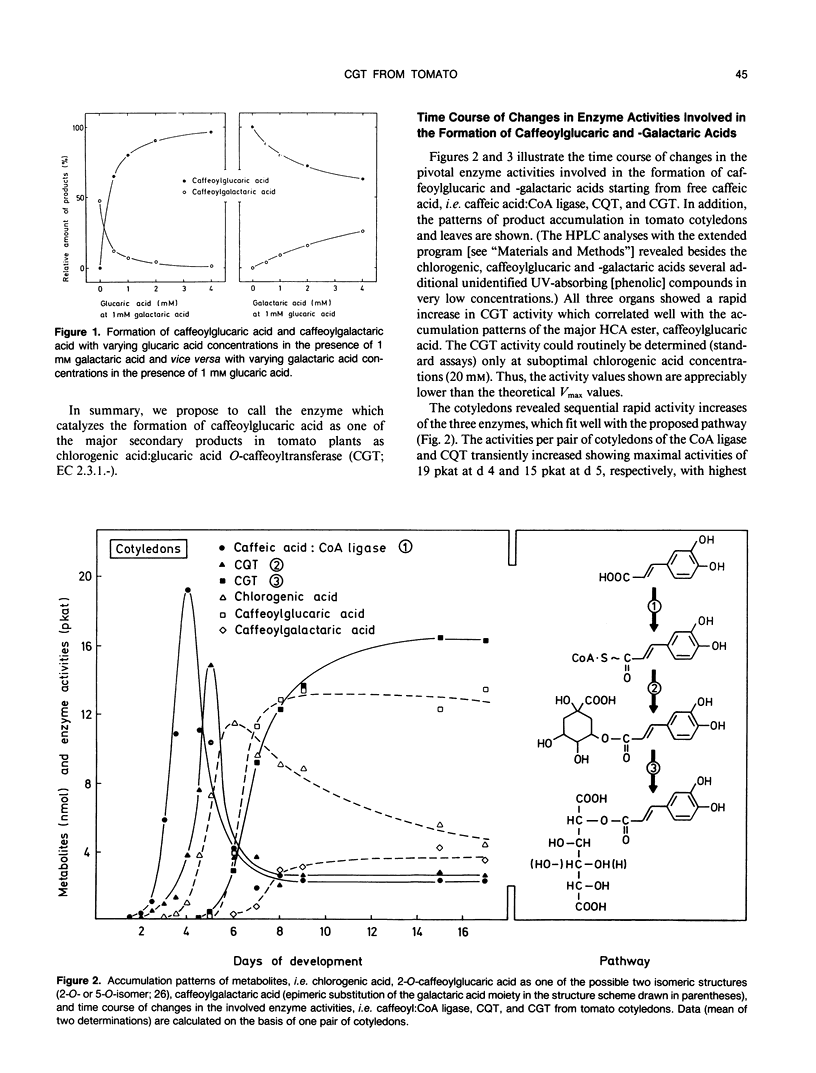
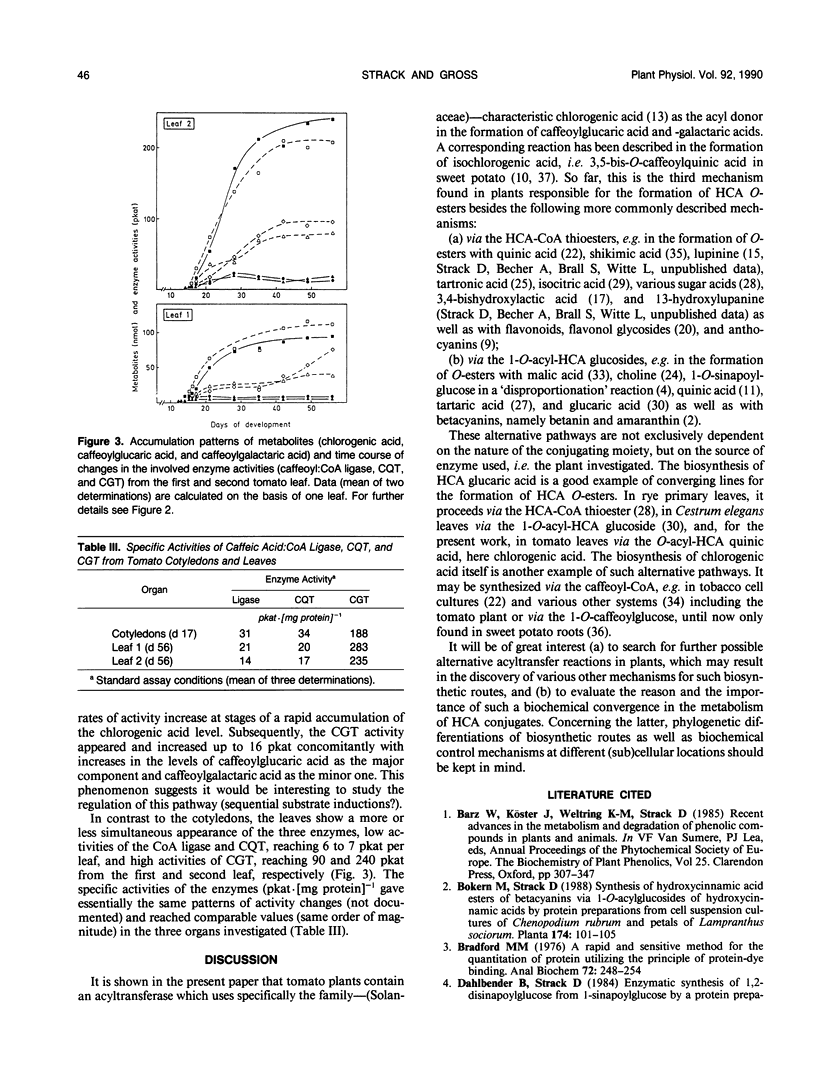
A novel acyltransferase from cotyledons of tomato (Lycopersicon esculentum Mill.), which catalyzes the transfer of caffeic acid from chlorogenic acid (5-O-caffeoylquinic acid) to glucaric and galactaric acids, was purified with a 2400-fold enrichment and a 4% recovery. The enzyme showed specific activities (theoretical Vmax per milligram of protein) of 625 nanokatals (caffeoylglucaric acid formation) and 310 nanokatals (caffeoylgalactaric acid formation). On sodium dodecyl sulfate-polyacrylamide gel electrophoresis it gave an apparent Mr of 40,000, identical to the value obtained by gel filtration column chromatography. Highest activity was found at pH 5.7, which was constant over a range of 20 to 120 millimolar K-phosphate. The isoelectric point of the enzyme was at pH 5.75. The reaction temperature optimum was at 38°C and the apparent energy of activation was calculated to be 57 kilojoules per mole. The apparent Km values were 0.4 millimolar for glucaric acid, 1.7 millimolar for galactaric acid, and with both acceptors as second substrates 20 millimolar for chlorogenic acid. The relative ratio of the Vmax/Km values for glucaric acid and galactaric acid was found to be 100:12. Substrate-competition experiments support the conclusion that one single enzyme is responsible for both the glucaric and galactaric acid ester formation with marked preference for glucaric acid. It is proposed that the enzyme be called chlorogenic acid:glucaric acid O-caffeoyltransferase (EC 2.3.1.-). The three caffeic acid-dependent enzyme activities involved in the formation of the glucaric and galactaric acid esters, the chlorogenic acid:glucaric acid caffeoyltransferase as the key activity as well as the caffeic acid:CoA ligase and the caffeoyl-CoA:quinic acid caffeoyltransferase as the preceding activities, were determined. The time course of changes in these activities were followed during development of the seedling in the cotyledons and growth of the young plant in the first and second leaf. The results from tomato seedlings suggest a sequential appearance of these enzymes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol. 1974;32:92–102. doi: 10.1016/0076-6879(74)32012-5. [DOI] [PubMed] [Google Scholar]
- Saylor M. H., Mansell R. L. Hydroxycinnamoyl: coenzyme A transferase involved in the biosynthesis of kaempferol-3-(p-coumaroyl triglucoside) in Pisum sativum. Z Naturforsch C. 1977 Sep-Oct;32(9-10):764–768. [PubMed] [Google Scholar]
- Strack D., Gross W., Wray V., Grotjahn L. Enzymic synthesis of caffeoylglucaric Acid from chlorogenic Acid and glucaric Acid by a protein preparation from tomato cotyledons. Plant Physiol. 1987 Mar;83(3):475–478. doi: 10.1104/pp.83.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöckigt J., Zenk M. H. Chemical syntheses and properties of hydroxycinnamoyl-coenzyme A derivatives. Z Naturforsch C. 1975 May-Jun;30(3):352–358. doi: 10.1515/znc-1975-5-609. [DOI] [PubMed] [Google Scholar]
- Stöckigt J., Zenk M. H. Enzymatic synthesis of chlorogenic acid from caffeoyl coenzyme A and quinic acid. FEBS Lett. 1974 Jun 1;42(2):131–134. doi: 10.1016/0014-5793(74)80769-6. [DOI] [PubMed] [Google Scholar]


