Abstract
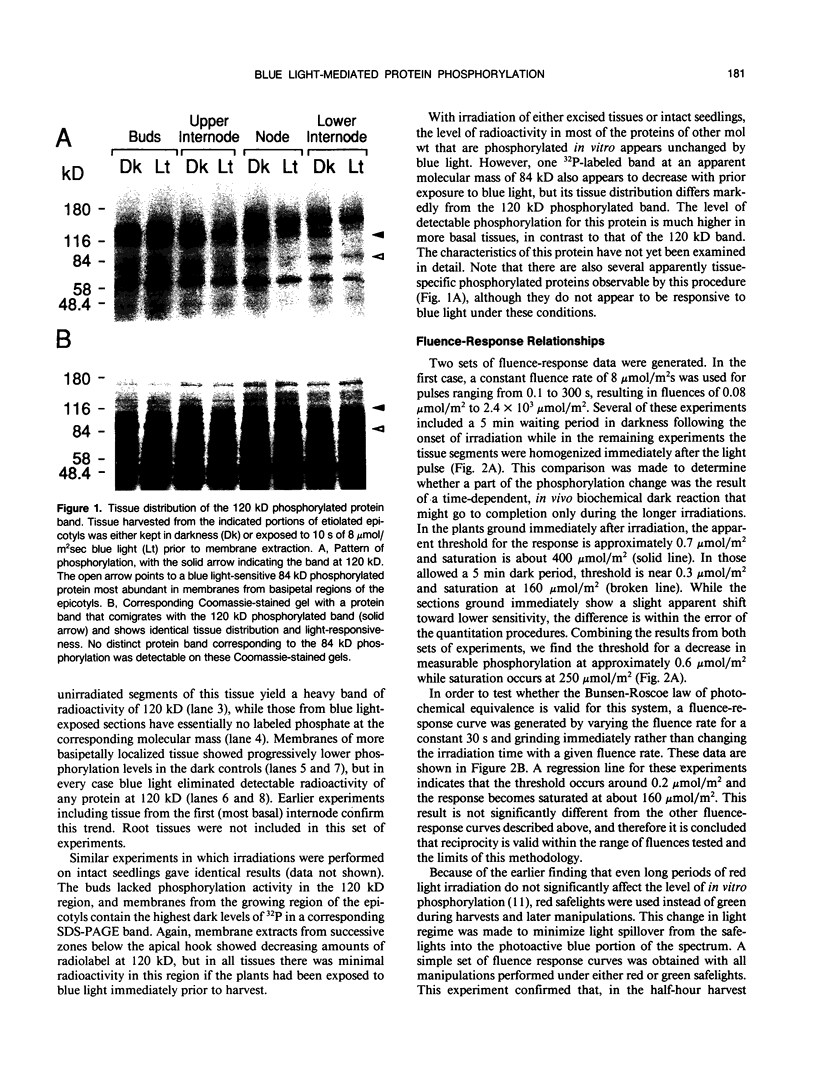
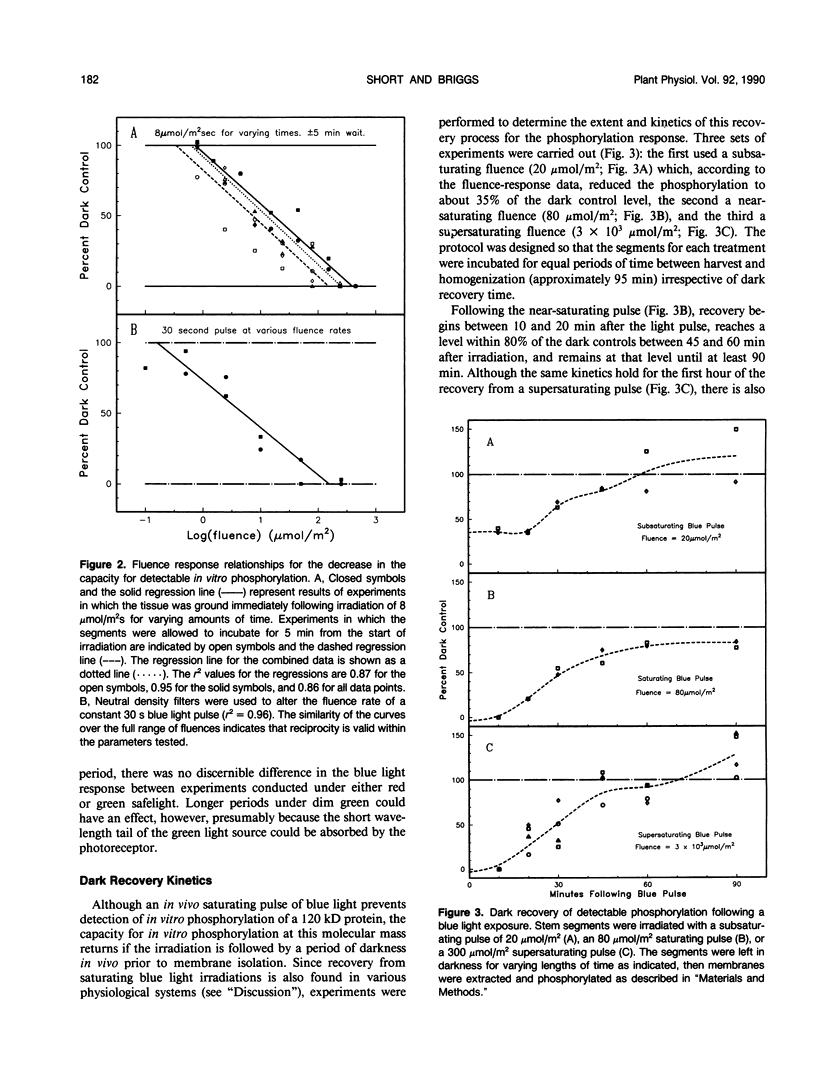
When crude microsomal membranes from apical stem segments of etiolated Pisum sativum L. cv Alaska are mixed in vitro with γ-[32P]ATP, a phosphorylated band of apparent molecular mass 120 kilodaltons can be detected on autoradiographs of sodium dodecyl sulfate electrophoresis gels. If the stem sections are exposed to blue light immediately prior to membrane isolation, this band is not evident. The response is observed most strongly in membranes from the growing region of the stem, but no 120 kilodalton radiolabeled band is detected in membranes from the developing buds. Fluence-response curves for the reaction show that the system responds to blue light above about 0.3 micromole per square meter, and the visible phosphorylation completely disappears above 200 micromoles per square meter. Reciprocity is valid for the system, because varying illumination time or fluence rate give similar results. If the stem segments are left in the dark following a saturating blue irradiation, the radio-labeled band begins to return after about 10 minutes and is as intense as that from the dark controls within 45 to 60 minutes. A protein that comigrates with the phosphorylated protein on polyacrylamide gels is also undetectable after saturating blue light irradiations. The fluence range in which the protein band disappears is the same as that for the disappearance of the phosphorylation band. Its dark recovery kinetics and tissue distribution also parallel those for the phosphorylation. In vitro irradiation of the isolated membranes also results in a phosphorylation change at that molecular mass, but in the opposite direction. Comparisons of the kinetics, tissue distribution, and dark recovery of the phosphorylation response with those published for blue light-mediated phototropism or rapid growth inhibition indicate that the phosphorylation could be linked to one or both of those reactions. However, the fluence-response relationships for the change in detectable phosphorylation match quite closely those reported for phototropism but not those for growth inhibition. Blue light has also been found to regulate the capacity for in vitro phosphorylation of a second protein. It has an apparent molecular mass of 84 kilodaltons and is localized primarily in basal stem sections.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs W. R. Light Dosage and Phototropic Responses of Corn and Oat Coleoptiles. Plant Physiol. 1960 Nov;35(6):951–962. doi: 10.1104/pp.35.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs W. R. Mediation of Phototropic Responses of Corn Coleoptiles by Lateral Transport of Auxin. Plant Physiol. 1963 May;38(3):237–247. doi: 10.1104/pp.38.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J. Kinetic separation of phototropism from blue-light inhibition of stem elongation. Photochem Photobiol. 1985;42(6):745–751. doi: 10.1111/j.1751-1097.1985.tb01642.x. [DOI] [PubMed] [Google Scholar]
- Cosgrove D. J. Rapid Suppression of Growth by Blue Light: OCCURRENCE, TIME COURSE, AND GENERAL CHARACTERISTICS. Plant Physiol. 1981 Mar;67(3):584–590. doi: 10.1104/pp.67.3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S., Short T. W., Ray P. M., Pratt L. H., Briggs W. R. Light-mediated changes in two proteins found associated with plasma membrane fractions from pea stem sections. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8003–8007. doi: 10.1073/pnas.85.21.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Ogawa T., Zeiger E. Kinetic properties of the blue-light response of stomata. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8019–8023. doi: 10.1073/pnas.82.23.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. G., Burg S. P. Red light enhancement of the phototropic response of etiolated pea stems. Plant Physiol. 1974 Mar;53(3):445–448. doi: 10.1104/pp.53.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M. J., Briggs W. R. Regulation of pea epicotyl elongation by blue light : fluence-response relationships and growth distribution. Plant Physiol. 1989 Jan;89(1):293–298. doi: 10.1104/pp.89.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K., Baskin T. I., Briggs W. R. Evidence for a phytochrome-mediated phototropism in etiolated pea seedlings. Plant Physiol. 1989 Feb;89(2):493–497. doi: 10.1104/pp.89.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warpeha K. M., Kaufman L. S. Blue-Light Regulation of Epicotyl Elongation in Pisum sativum. Plant Physiol. 1989 Feb;89(2):544–548. doi: 10.1104/pp.89.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]