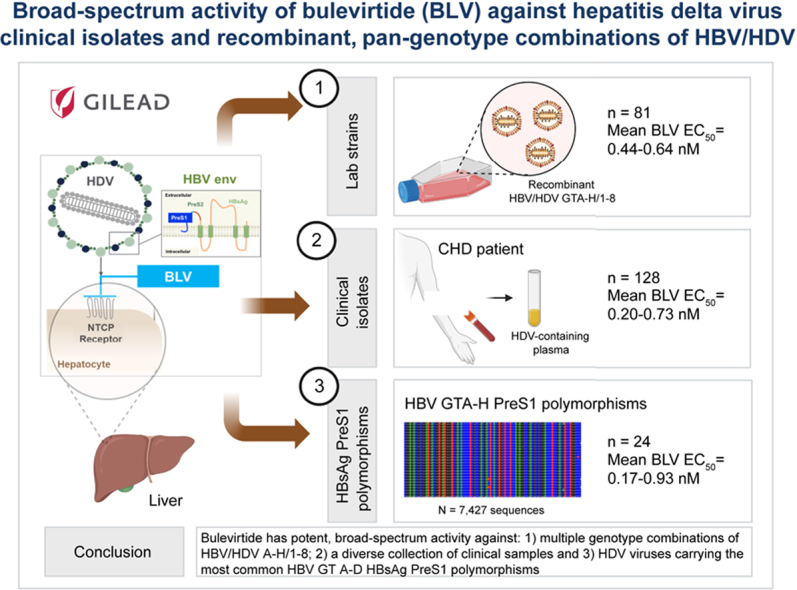
Abstract
Background & Aims
Bulevirtide (BLV) is a small lipopeptide agent that specifically binds to the sodium taurocholate cotransporting polypeptide (NTCP) bile salt transporter and HBV/HDV receptor on the surface of human hepatocytes and inhibits HDV and HBV entry. As a satellite virus of HBV, HDV virions are formed after assembly of HDV RNA with the HBV envelope proteins (HBsAg). Because both viruses exist as eight different genotypes, this creates a potential for high diversity in the HBV/HDV combinations. To investigate the sensitivity of various combinations of HBV/HDV genotypes to BLV, clinical and laboratory strains were assessed.
Methods
For the laboratory strains, the different envelopes from HBV genotypes A through H were combined with HDV genotypes 1–8 in cotransfection assays. Clinical plasma isolates were obtained from clinical studies and academic collaborations to maximise the diversity of HBV/HDV genotypes tested.
Results
The mean BLV EC50 against HDV laboratory strains ranged from 0.44 to 0.64 nM. Regardless of HBV and HDV genotypes, the clinical isolates showed similar sensitivities to BLV with mean values that ranged from 0.2 to 0.73 nM.
Conclusions
These data support the use of BLV in patients infected with any HBV/HDV genotypes.
Impact and implications
This study describes the potent activity of BLV against multiple laboratory strains spanning all HBV/HDV A–H/1–8 genotype combinations and the most diverse collection of HDV clinical samples tested to date, including HBV/HDV genotype combinations less frequently observed in the clinic. Overall, all isolates and laboratory strains displayed similar in vitro nanomolar sensitivity to BLV. This broad-spectrum antiviral activity of BLV has direct implications on potential simplified treatment for any patient infected with HDV, regardless of genotype, and supports the new 2023 EASL Clinical Practice Guidelines on HDV that recommend antiviral treatment for all patients with CHD.
Keywords: hepatitis delta virus, hepatitis, Bulevirtide
Graphical abstract
Highlights
-
•
Bulevirtide is a potent inhibitor against a diverse collection of clinical samples from patients with CHD in vitro.
-
•
Bulevirtide inhibits all recombinant genotype combinations of HBV/HDV A–H/1–8 with similar nanomolar potency.
-
•
Bulevirtide is active against HDVs carrying all common HBV A–D HBsAg PreS1 polymorphisms.
-
•
Broad-spectrum activity of bulevirtide supports its use for treatment of all patients infected with HDV, regardless of genotype.
Introduction
Chronic hepatitis D (CHD) is the most severe form of viral hepatitis, with rapid progression of liver-related diseases and high rates of development of hepatocellular carcinoma.1,2 At least 12 million people worldwide have experienced HDV infection, although the exact prevalence remains controversial because of unreliable assessment of the infection and the lack of real-world surveillance.3 Recent data have shown that HDV continues to spread in low-income countries with a prevalence that has remained stable or increased in certain regions.4 Therefore, the real number of patients infected with HDV may have been severely underestimated.5 With limited therapeutic interventions available, CHD disease remains an urgent but unmet medical need.6,7
HDV, the causative agent of CHD, is a defective small RNA virus that requires the HBsAg for its complete infection and transmission.[8], [9], [10], [11] Eight genotypes of HDV exist, with genotype 1 (HDV-1) being the most common globally and in North America.8 Whereas HDV-1 is the most prevalent and distributed worldwide, HDV-2 is restricted to Southeast Asia.12 HDV-3, the most distantly related genotype, is common in northern areas of South America, whereas HDV-4 is found in Japan and China.12 HDV-5, -6, -7, and -8 are prevalent in African countries.12
HDV RNA can replicate in the liver in the absence of HBV.11 This tissue specificity is based on the hepatocyte-specific expression of the sodium taurocholate cotransporting polypeptide (NTCP) receptor, a bile acid transporter that also acts as an HBV/HDV receptor.13 For secretion and de novo entry, newly synthesised HDV RNA/protein complexes require all three forms of HBsAg, namely, large (L), middle (M), and small (S), which collectively compose the HBV envelope.11 Notably, HBV genotypes have different geographical distributions: genotype A (GTA), Sub-Saharan Africa and Asia; GTB and GTC, Asia; GTD, Asia, Africa, and Europe; GTE, Sub-Saharan Africa; and GTF–H, Latin America.14 In HDV-infected patients, however, the distribution of HBV genotypes has not been thoroughly investigated.
Synthetic PreS1 lipopeptides from diverse HBV genotypes differ in their ability to interfere with the binding of HBV to NTCP.15 This argues that HDV particles containing envelope proteins from different HBV genotypes might vary in their infection efficacy, spreading abilities, and pathogenesis in patients. Therefore, the study of the multiple HBV/HDV combinations and their susceptibilities to treatment remains of great importance to assess coverage of circulating HDV viruses.16
Bulevirtide (BLV), formerly known as Myrcludex B, is a 47-amino acid, N-terminally myristoylated, HBV large envelope (L) protein–derived lipopeptide that binds specifically to NTCP and acts as a potent, highly selective entry inhibitor of HDV and HBV into hepatocytes.17,18 In April 2023, BLV 2 mg, administered as a daily s.c. injection, was granted full marketing authorization by the EMA for the treatment of CHD and compensated liver disease. Several clinical studies have shown its safety and efficacy at reducing HDV viral load and normalising alanine aminotransferase levels.[19], [20], [21], [22], [23], [24] Unfortunately, limited data on the efficacy of BLV on different HBV/HDV genotype combinations exist, as most participants enrolled in these studies were HBV/HDV D/1, with some A/1 and a few E/5. In the clinic, it is unlikely that all possible combinations of HBV/HDV genotypes exist, making coverage evaluation of BLV for all potential genotype combinations unattainable by analysing patient samples only.
In this study, the in vitro antiviral activity of BLV against laboratory-derived HDV-1 to HDV-8 viruses enveloped with HBV GTA–H (81 recombinant viruses) was assessed. Similarly, a panel of 128 clinical plasma isolates of diverse combinations of HDV/HBV genotypes were tested for their inhibitory properties to BLV.
Materials and methods
Sensitivity assays of BLV were performed at Gilead Sciences, Inc. (Foster City, CA, USA).
Clinical isolates
A panel of patient plasma samples from 115 HDV-infected participants enrolled in Gilead-sponsored clinical studies and treated with BLV (studies MYR204 [ClinicalTrials.gov, NCT03852433; EudraCT 2019-001485-15]25 and MYR301 [ClinicalTrials.gov, NCT03852719; EudraCT 2019-001213-17])26 were tested for in vitro sensitivity against BLV. In addition, 13 plasma samples from participants infected with HDV from less common HBV genotypes (GTA, GTB, GTC, and GTE) were studied for their sensitivity to BLV. These samples come from participants from six different countries of birth and were obtained through external collaborators.
All studies were conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulatory requirements. All patients provided written informed consent.
Compounds
BLV was synthesised by Gilead.
Cell culture
HuH7 cells were obtained from ReBLikon GmbH. Cells were maintained in DMEM/F12 supplemented with 10% heat-inactivated FBS (Hyclone) and 1× penicillin–streptomycin–glutamine (Life Technologies).
Primary human hepatocytes (PHHs) were obtained from BioIVT. Cells were plated with William’s Medium E (Thermo Scientific) supplemented with Hepatocyte Plating Supplement Pack and maintained in William’s Medium E with 2% FBS, 1.5% DMSO (Sigma-Aldrich), and Hepatocyte Maintenance Supplement Pack (Gibco). All assays in this report were performed using a single PHH donor (KBK).
Construction of HBV envelope expression constructs
HBV envelope for GTA–H
The eight constructs carrying HBV GTA–H envelopes are gifts from Dr. Stephan Urban. In brief, the HB2.7 subgenomic fragments were amplified by PCR from eight constructs containing HBV GTA–H sequences. The accession numbers for these HBV GTA–H sequences are MN645903–MN645910. The HB2.7 subgenomic fragments were then inserted into lentiviral production plasmid pLX304. Detailed information about these constructs is described by Wang et al.16
HBV envelope polymorphisms
A total of 7,427 sequences from public databases and internal sources comprising HBV GTA–H (6,768 sequences were GTA–D) were analysed to identify polymorphisms within the BLV region of HBV envelope for each genotype. For GTA–D, HBV envelope polymorphism constructs were designed to cover the most common polymorphisms of the BLV region. A total of 24 HBV envelope polymorphism constructs were generated: two for GTA, 10 for GTB, 10 for GTC, and two for GTD. Table S2 shows the sequence alignment of representative sequences from HBV GTA–H with the BLV sequence. The HBV envelope constructs containing polymorphic changes were generated by synthesising a 2.7-kb HBV fragment containing HBV envelope from clinical isolates across GTA–D and cloning into pLX304 plasmids.
HDV constructs
The 1.1-fold antigenomic cDNA sequences of HDV-1 (accession number U81989), HDV-2 (accession number X60193), HDV-3 (accession number L22063), HDV-4 (accession number AB118847), HDV-5 (accession number AM183331), HDV-6 (accession number AM183329), HDV-7 (accession number AM183333), and HDV-8 (accession number AM183327) were gifts from Dr. Stephan Urban. More detailed information about these constructs is described by Wang et al.16
Transient transfection
HDVs were produced by cotransfection of HuH7 cells with the corresponding HDV plasmids (pcDNA3.1-HDV-1 to pcDNA3.1-HDV-8) and HBV plasmids pLX304-HB2.7-A to pLX304-HB2.7-H using Lipofectamine 3000 as previously described.16 In brief, HuH7 cells were seeded in T75 flasks at 4 × 106 cells 1 day before transfection. Sixteen hours after transfection, cells were washed with PBS and fresh medium. Media were replaced every second or third day and collected at Day 8 or 13 for PHH infections. Quantitation of HDV RNA in the supernatant of cells after transfection of an in-house HDV-1 laboratory strain carrying the HBV GTB envelope (BB-1) was used as a control for transfection efficiency in all experiments.
HDV RNA quantification
HDV RNA was extracted from the supernatant medium using the QIAamp viral RNA Mini KIt (QIAGEN, Cat# 52904) according to the manufacturer’s instructions. In brief, the viral supernatant was first lysed under highly denaturing conditions and loaded onto the QIAamp Mini spin column. The RNA bound to the membrane and washed with two different wash buffers to remove protein, nucleases, and other contaminants and inhibitors. RNA was eluted in nuclease-free water and proceeded to the next step or stored at -80 °C freezer for later use.
RNA then was treated with DNase I (Thermo Fisher, Cat# 18068015) to remove any residual DNA. Quantitative real-time PCR for HDV RNA was performed using TaqMan™ Fast Virus 1-Step Master Mix (Thermo Fisher, Cat# 4444434) on a QuantStudio Fast Real-Time PCR system (Thermo Fisher). Amplification primers are as follows: HDV forward, GCGCCGGCYGGGCAAC; HDV reverse, TTCCTCTTCGGGTCGGCATG; and HDV probe, CGCGGTCCGACCTGGGCATCCG. RNA copies per millilitre were calculated against a standard curve.
EC50 determination
EC50 values were determined by seeding PHHs in 96-well plates at a density of 60,000 cells/well. One day after seeding, PHHs were pretreated with 10 replicate dilutions of BLV for 0.5 to 1 h before virus inoculation. After BLV incubation, PHHs were infected with 2 μl of clinical plasma or 40 μl of an HDV laboratory strain, unless otherwise indicated, in the presence of 4% PEG8000 (Sigma-Aldrich) and 1.5% v/v DMSO and kept in culture for 5 days. An in-house HDV-1 laboratory strain carrying the HBV GTB envelope (BB-1) was included as a control. HDV antigen (HDAg)-positive cells were stained and quantified as described in the HDAg Staining section.
HDAg staining
Immunofluorescence staining against HDAg was performed at Day 5 post infection. In brief, PHH cells cultured in white, collagen-coated, clear 96-well plates were fixed in 4% paraformaldehyde for 30 min, followed by 30 min of incubation in blocking buffer (PBS, 0.3% Triton, 3% BSA, 10% FBS). After washing with PBS, cells were incubated for 1 h with an anti-HDAg mouse monoclonal antibody (Gilead) diluted in incubation buffer (PBS, 0.3% Triton, 3% BSA). Then, cells were washed and incubated with Hoescht and secondary goat antimouse (Invitrogen) antibody for 1 h. After extensive washing, the percentage of HDAg-positive cells compared with total cell numbers was calculated using software HSC Studio Cellomics Scan (version 6.6.2, Thermo Scientific).
Statistical analysis
Data were calculated using nonlinear regression analysis GraphPad Prism 8 software package (IDBS, Emeryville, CA, USA). The concentration of BLV for which there was 50% decrease in HDV antigen production was expressed as a mean EC50 value from at least two independent experiments.
Results
Establishment and optimisation of an assay for robust production of infectious HDV viral particles of diverse genotypes
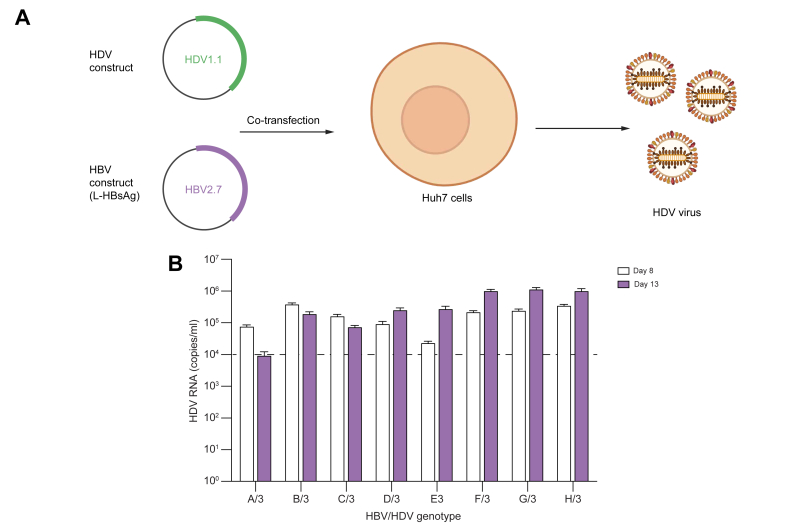
Genotype-specific studies of the infectivity of HDV-1 to HDV-8 enveloped with L-HBsAg from HBV GTA–H have been described before using HuH7-NTCP cells.16 In this assay, HuH7-NTCP cells were plated into large flasks and cotransfected with a mixture of HDV and HBV plasmids. Cultures were incubated and supernatants were harvested and tittered on Days 8 and 13 to study the kinetics of production of infectious HDV (Fig. 1). As shown in Fig. 1B using HDV-3, viral titres were generally similar or increased slightly from Day 8 to Day 13. Regardless, by Day 8, all combinations of HDV genotypes with HBV GTA–H had produced enough infectious virus (∼104 copies/ml) to determine their sensitivity to BLV. Based on this information, all transfections were carried out for 8 days unless otherwise indicated.
Fig. 1.
Production of infectious HDV.
(A) Cells were seeded and used to produce HDVs by cotransfection with HDV and HBV plasmids. Sixteen hours after transfection, cells were washed, and supernatant medium was refreshed and collected at Days 8 or 13 and cleared with centrifugation before HDV RNA quantitation. (B) Supernatants were collected, and RNA was extracted and quantified using qRT-PCR. Production of HDV genotype 3 cotransfected with PreS1 from HBV GTA–H is shown as a representative example. An average of three replicate experiments is shown. Error bars indicate SD. BLV, bulevirtide; GTA–H, genotypes A–H; HDAg, HDV antigen; qRT-PCR, quantitative real-time PCR.
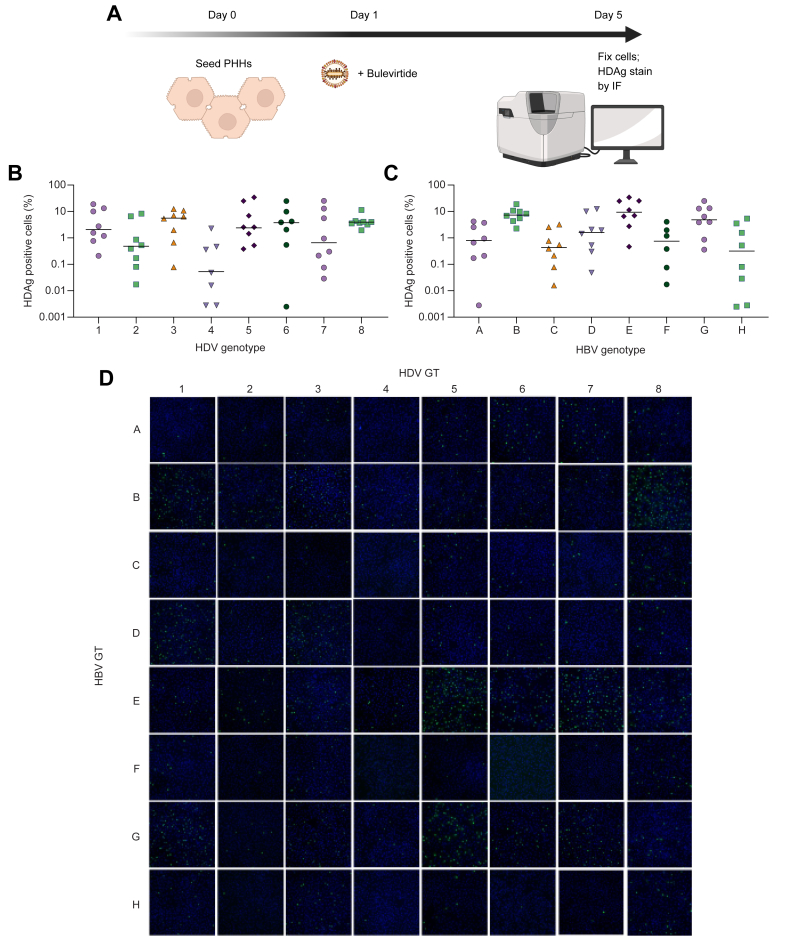
Establishment of an HDV viral infectivity assay using PHHs
Viruses generated after cotransfection and collected on Day 8 were used to infect PHHs. Five days later, PHH monolayers were fixed, and HDAg was quantified by immunofluorescence (Fig. 2A). Combinations of HBV GTA–H with HDV-1 to HDV-8 resulted in differences in rates of HDAg positivity higher than ∼100-fold depending on the particular combination (Fig. 2B–D and Table S1). However, most combinations displayed sufficient infectivity to assess their sensitivity to BLV, even with infectivity values as low as 0.03% (HBV/HDV H/7). Specifically, HBV/HDV genotype combinations E/7, E/5, and B/1 had the highest percentage of HDAg positivity in PHHs, with values of 25%, 20%, and 19%, respectively. On the contrary, HBV/HDV genotype combinations F/2 and H/7 had the lowest measurable values of 0.02% and 0.03%, respectively. Seven rare HBV/HDV genotype combinations resulted in no measurable fluorescence signal in the PHH assay: A/4, C/4, D/4, F/4, H/4, F/6, and H/6. Overall, a measurable percentage of cells positive for HDAg staining could be determined for 89% (57/64) of viruses generated.
Fig. 2.
In vitro HDV phenotypic assay in PHHs.
(A) PHHs were seeded and pretreated with several dilutions of BLV before virus inoculation. Then, they were infected with clinical plasma or an HDV laboratory strain and kept in culture for 5 days. At that time, IF staining against HDAgs was performed, and the percentage of positive cells was calculated using software HCS Studio Cellomics Scan. (B) Percentage of HDAg-positive cells by infecting HDV genotypes 1–8. Each data point represents the average of two independent experiments. Bars show median values. (C) Percentage of HDAg-positive cells stratified by infecting HBV genotypes A–H tested. Each data point represents the average of two independent experiments. Bars show median values. (D) Representative fluorescence images of HDAg staining on Day 5 post infection for all HBV/HDV genotype combinations. Each assay was performed at least in two independent experiments. BLV, bulevirtide; GT, genotype; HDAg, HDV antigen; IF, immunofluorescence; PHH, primary human hepatocyte.
Sensitivity to BLV of HDV laboratory strains comprising HDV-1 to HDV-8 enveloped with HBV GTA–H
To determine the BLV sensitivity of HDV-1 to HDV-8 enveloped with HBV GTA–H, viruses were generated in vitro and treated with BLV in the PHH infection assay as described above. Despite differences in the infectivity (percentage of HDAg-positive cells) for the various combinations, BLV potently inhibited HDV infection in all the 57 HDV/HBV combinations that produced enough infectious virus in this assay. Similar EC50 values across genotypes were observed (Table 1). However, seven laboratory strains resulted in assay failure as no measurable signal for HDAg staining was detected: HDV-4 with HBV GTA, GTC, GTD, GTF, and GTH envelopes and HDV-6 with HBV GTF and GTH envelopes. Overall, BLV EC50 values could be determined for 89% (57/64) of the viruses generated (Table 1).
Table 1.
Antiviral activity of BLV against HDV-1 to HDV-8 enveloped with HBV GTA–H.
| HBV GT | BLV EC50 (nM) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HDV-1 | HDV-2 | HDV-3 | HDV-4 | HDV-5 | HDV-6 | HDV-7 | HDV-8 | HBV mean | HBV median | |
| GTA | 0.65 ± 0.03 | 0.44 ± 0.06 | 0.32 ± 0.05 | n.a. | 0.52 ± 0.03 | 0.48 ± 0.08 | 0.59 ± 0.11 | 0.43 ± 0.09 | 0.49 | 0.48 |
| GTB | 0.90 ± 0.29 | 0.53 ± 0.01 | 0.50 ± 0.06 | 0.71 ± 0.24 | 0.84 ± 0.04 | 0.66 ± 0.03 | 0.82 ± 0.03 | 0.73 ± 0.06 | 0.71 | 0.72 |
| GTC | 0.46 ± 0.17 | 0.59 ± 0.13 | 0.32 ± 0.06 | n.a. | 0.32 ± 0.02 | 0.49 ± 0.02 | 0.46 ± 0.10 | 0.93 ± 0.24 | 0.51 | 0.46 |
| GTD | 0.49 ± 0.04 | 0.23 ± 0.06 | 0.36 ± 0.06 | n.a. | 0.52 ± 0.05 | 0.33 ± 0.13 | 0.38 ± 0.12 | 0.38 ± 0.05 | 0.38 | 0.38 |
| GTE | 0.85 ± 0.09 | 0.39 ± 0.04 | 0.54 ± 0.05 | 0.65 ± 0.01 | 0.76 ± 0.13† | 0.65 ± 0.03† | 0.80 ± 0.05 | 0.64 ± 0.11 | 0.66 | 0.65 |
| GTF | 0.28 ± 0.09 | 0.38∗ | 0.30 ± 0.11 | n.a. | 0.34 ± 0.03 | n.a. | 0.24 ± 0.02 | 0.52 ± 0.10 | 0.34 | 0.32 |
| GTG | 0.57 ± 0.09 | 0.68 ± 0.07 | 0.76 ± 0.01 | 0.56 ± 0.01 | 0.73 ± 0.08† | 0.67 ± 0.18 | 0.86 ± 0.02 | 0.59 ± 0.01 | 0.68 | 0.68 |
| GTH | 0.45 ± 0.03 | 0.61 ± 0.18 | 0.39 ± 0.04 | n.a. | 0.34 ± 0.07 | n.a. | 0.31 ± 0.06 | 0.56 ± 0.12 | 0.44 | 0.42 |
| HDV mean | 0.58 | 0.48 | 0.44 | 0.64 | 0.55 | 0.55 | 0.56 | 0.60 | ||
| HDV median | 0.53 | 0.49 | 0.38 | 0.65 | 0.52 | 0.57 | 0.53 | 0.58 | ||
HBV/HDV GT combinations were produced by transfection of HuH7 cells and phenotyped against BLV in replicate experiments in a PHH infectivity assay. Mean and median values are shown.
BLV, bulevirtide; GT, genotype; GTA–H, genotypes A–H; HDV-1 to HDV-8, HDV genotypes 1–8; n.a., not applicable owing to low infectivity; PHH, primary human hepatocyte.
n = 1 owing to very low infectivity.
EC50 was averaged in two independent experiments with 40 and 4 μl of input viral inoculum.
The mean EC50 of BLV against HDV-1 to HDV-8 enveloped with HBsAg from HBV GTA-D ranged from 0.38 to 0.71 nM. Similarly, for HDV-1 to HDV-8 enveloped with HBsAg from HBV GTE–H, the mean EC50 of BLV ranged from 0.34 to 0.68 nM.
Across HDV-1 to HDV-8 enveloped with HBV GTA–H envelopes, the mean EC50 of BLV ranged from 0.44 to 0.64 nM. Taken together, BLV showed similarly potent activity against all eight HDV genotypes and eight HBV genotypes with a very narrow range of EC50 values.
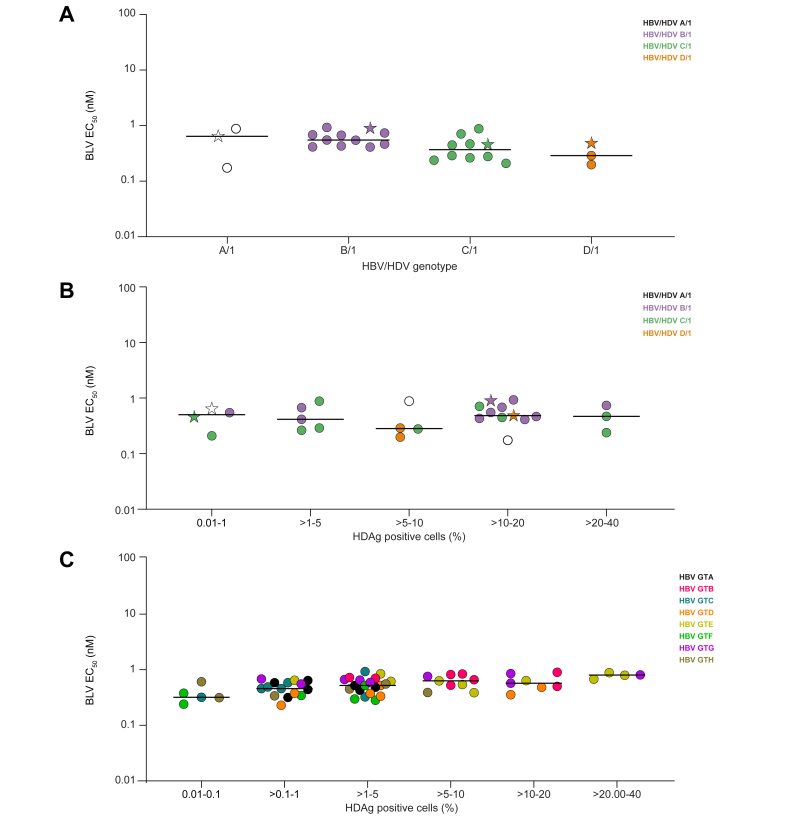
Activity of BLV against HDV-1 with representative PreS1 sequences from HBV GTA–H and the most common polymorphisms in HBV GTA–D
To determine the impact of PreS1 polymorphisms on sensitivity to BLV, polymorphic analyses of 7,427 HBV sequences from GTA–H obtained from public databases and Gilead internal sources were conducted. A high degree of conservation of the 47-amino acid-long peptide sequence that corresponds to the BLV sequence was found, with a remarkable conservation of the sequence from positions 9-NPGLFFP-15, which is crucial for binding. Table S2 shows the alignment of representative sequences from HBV GTA–H with the BLV sequence. Among the most common genotypes GTA–D, GTC was the closest to the BLV sequence with only one amino acid change (K57Q). GTA and GTB had four and six amino acid changes, respectively, and GTD had eight compared with the BLV sequence. Importantly, the sensitivity to BLV of HDVs containing these sequences were similar for the common GTA–D with EC50 values ranging from 0.46 to 0.90 nM (Table S2). The remaining HBV GTE–H had between eight and nine amino acid changes compared with the BLV sequence; however, their EC50 values were within assay variation and remained similar to those of the other genotypes with values ranging from 0.28 to 0.85 nM. Overall, no significant differences across different genotypes were observed for any of the viruses tested.
Next, a sequence analysis was conducted to determine the most common amino acid variations in the PreS1 sequence found in clinical isolates from HBV GTA–D (Table S3). To further evaluate the activity of BLV against these multiple variants identified, in vitro HDV-1 laboratory strains were generated. Given that HDV clinical isolates with HBV GTB and GTC envelopes were underrepresented in our BLV clinical isolates, a bigger subset of HBV GTB and GTC envelope constructs compared with GTA and GTD were generated with 10 HBV GTB and 10 HBV GTC envelope constructs each, as well as two HBV GTA and GTD envelope constructs each. Regardless of polymorphisms, the mean EC50 of BLV for HDV-1 enveloped with multiple strains of HBV GTA, GTB, GTC, and GTD were 0.57, 0.61, 0.42, and 0.32 nM, respectively, very similar to those obtained for HDVs enveloped with PreS1 containing representative sequences of HBV GTA, GTB, GTC, and GTD (0.65, 0.90, 0.46, and 0.49 nM, respectively) (Table 2 and Fig. 3A). Thus, BLV maintained similar and potent inhibition of HDV-1 enveloped with HBV GTA–D laboratory strains containing the major polymorphisms in PreS1.
Table 2.
Antiviral activity of BLV against multiple strains of HBV GTA–D enveloped with HDV-1.
| HBV GTA |
HBV GTB |
HBV GTC |
HBV GTD |
|||||
|---|---|---|---|---|---|---|---|---|
| HBV construct | EC50 (nM) | HBV construct | EC50 (nM) | HBV construct | EC50 (nM) | HBV construct | EC50 (nM) | |
| EC50 (nM) of individual viral strains | GTA∗ | 0.65 ± 0.03 | GTB∗ | 0.90 ± 0.29 | GTC∗ | 0.46 ± 0.17 | GTD∗ | 0.49 ± 0.04 |
| GTA-1 | 0.17 ± 0.05 | GTB-1 | 0.47 ± 0.06 | GTC-1 | 0.47 ± 0.07 | GTD-1 | 0.29 ± 0.03 | |
| GTA-2 | 0.88 ± 0.10 | GTB-2 | 0.74 ± 0.19 | GTC-2 | 0.21 ± 0.01 | GTD-2 | 0.20 ± 0.01 | |
| – | – | GTB-3 | 0.55 ± 0.02 | GTC-3 | 0.71 ± 0.30 | – | – | |
| – | – | GTB-4 | 0.42 ± 0.19 | GTC-4 | 0.24 ± 0.01 | – | – | |
| – | – | GTB-5 | 0.41 ± 0.05 | GTC-5 | 0.45 ± 0.08 | – | – | |
| – | – | GTB-6 | 0.93 ± 0.01 | GTC-6 | 0.28 ± 0.05 | – | – | |
| – | – | GTB-7 | 0.67 ± 0.04 | GTC-7 | 0.88 ± 0.10 | – | – | |
| – | – | GTB-8 | 0.55 ± 0.38 | GTC-8 | 0.29 ± 0.04 | – | – | |
| – | – | GTB-9 | 0.68 ± 0.25 | GTC-9 | n.a. | – | – | |
| – | – | GTB-10 | 0.43 ± 0.07 | GTC-10 | 0.26 ± 0.11 | – | – | |
| Mean EC50 (nM) | GTA | 0.57 | GTB | 0.61 | GTC | 0.42 | GTD | 0.33 |
| Median EC50 (nM) | GTA | 0.65 | GTB | 0.55 | GTC | 0.37 | GTD | 0.29 |
HBVs/HDVs were produced by transfection of HuH7 cells and were tested for their sensitivity to BLV in PHHs. Mean and median values from replicate experiments are shown.
BLV, bulevirtide; GTA–D, genotypes A–D; HDV-1, HDV genotype 1; n.a., not applicable owing to low infectivity; PHH, primary human hepatocyte.
Representative sequences for each genotype.
Fig. 3.
Relationship between HBV/HDV genotype combination, HDAg positivity, and in vitro sensitivity to BLV in laboratory strains.
(A) Median BLV EC50 values for HDV-1 enveloped with representative sequences of HBV PreS1 GTA–D (stars) or the most common polymorphisms found in clinical samples. Each data point represents the average of two independent experiments. Bars show median values. (B) Median BLV EC50 values vs. percentage of HDAg-positive cells for HDV-1 enveloped with representative sequences of HBV PreS1 GTA–D (stars) or the most common polymorphisms found in clinical samples. Each data point represents the average of two independent experiments. Bars show median values. (C) Median BLV EC50 values as a function of the percentage of HDAg-positive cells for all HBV/HDV combinations tested. Each data point represents the average of at least two independent experiments. Bars show median values. BLV, bulevirtide; GTA–H, genotypes A–H; HDAg, HDV antigen; HDV-1, HDV genotype 1.
Analysis of the relationship between infectivity and sensitivity to BLV of HDV laboratory strains
Next, the relationship between the viral infectivity (percentage of HDAg-positive PHHs) of all HDV laboratory strains generated containing all the combinations of HBV/HDV and their in vitro sensitivity to BLV was investigated. As shown in Fig. 3, regardless of the genotype or their infectivity, all laboratory strains showed similar sensitivity to BLV (Fig. 3C). Similarly, when HDVs containing the most common polymorphisms observed in the PreS1 region of HBV GTA–D were investigated for their sensitivity to BLV, tight EC50 values similar to those of the corresponding HBV representative sequences for each genotype (denoted as stars) were observed (Fig. 3B).
In summary, sensitivity to BLV was not affected by either the HBV PreS1 sequence in the envelope of the HDVs tested or their infectivity in PHHs, with EC50 values that ranged between 0.17 and 0.93 nM (Table 2).
Establishment of an HDV phenotyping assay for clinical samples from patients with CHD
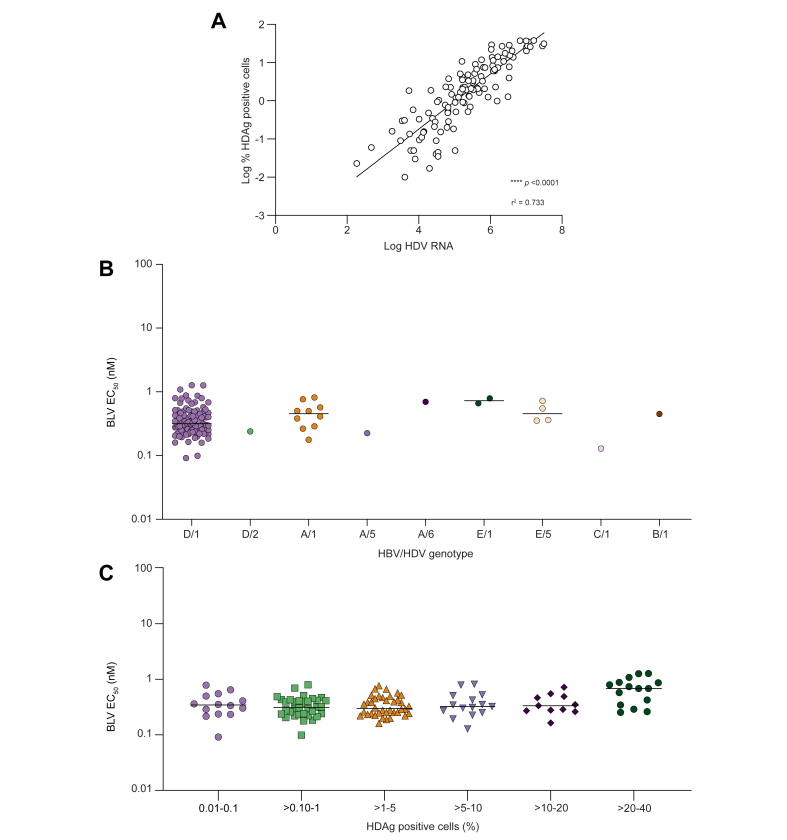
Plasma samples from participants with CHD were analysed for their in vitro infectivity and sensitivity to BLV in an assay similar to the one developed for testing HDV laboratory strains. In brief, 2 μl of patient plasma was used to infect a monolayer of freshly plated PHH cells. Five days later, cells were fixed and stained for HDAg, and the percentage of infected cells was calculated using fluorescent cell imaging. The assay showed robust infection of samples with high reproducibility of infection and EC50 values (data not shown). Importantly, there was a significant statistical correlation (Pearson correlation; p <0.0001, r2 = 0.733) between levels of PHH infection (percentage of HDAg-positive cells) and levels of HDV RNA, suggesting that this faster image-based assay could be used as a surrogate for HDV replication and infectivity and to determine sensitivity to BLV (Fig. 4A).
Fig. 4.
Effect of HBV/HDV genotype combination, HDAg positivity, and HDV viral load on in vitro sensitivity to BLV in clinical samples.
(A) Pearson correlation of HDAg-positive cells and HDV RNA (viral load) for 126/128 (98%) clinical samples described in this study (p <0.0001; r2 = 0.733). (B) Median BLV EC50 values plotted by HBV/HDV genotype combination. Each data point represents the average of at least two independent experiments. Bars show median values. (C) Median BLV EC50 values stratified by percentage of HDAg positivity for all clinical samples tested. Each data point represents the average of at least two independent experiments. Bars show median values. BLV, bulevirtide; HDAg, HDV antigen.
Activity of BLV against clinical isolates of various HBV/HDV genotypes from participants with CHD
The activity of BLV against 128 clinical isolates was evaluated in the PHH infectious system described above. The majority of clinical isolates tested (n = 107) were HBV/HDV D/1 and within a narrow range of the mean EC50 of 0.39 nM (Fig. 4B and Table 3). Ten participants were HBV/HDV A/1 with a mean EC50 of 0.47 nM. Four participants were infected with HBV/HDV E/5, and the clinical isolates from these participants were sensitive to BLV with a mean EC50 of 0.50 nM (Fig. 4B and Table 3). The patient isolates from the HBV/HDV combinations B/1, C/1, E/1, A/6, D/2, and A/5 were also tested, with mean EC50 values that ranged from 0.20 to 0.73 nM (Fig. 4B and Table 3).
Table 3.
Antiviral activity of BLV against HDV clinical isolates at baseline.
| HBV/HDV GT |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| BLV | D/1 (n = 107) | A/1 (n = 10) | E/5 (n = 4) | C/1 (n = 1) | E/1 (n = 2) | A/6 (n = 1) | B/1 (n = 1) | D/2 (n = 1) | A/5 (n = 1) |
| Mean EC50 ± SD (nM) | 0.39 ± 0.22 | 0.47 ± 0.21 | 0.50 ± 0.18 | 0.2 ± 0.08 | 0.73 ± 0.09 | 0.70 | 0.45 | 0.24 | 0.23 |
| Median | 0.32 | 0.46 | 0.46 | 0.18 | 0.73 | 0.70 | 0.45 | 0.24 | 0.23 |
| Range (min–max) | (0.09–1.27) | (0.18–0.64) | (0.35–0.72) | n.a. | (0.66–0.79) | n.a. | n.a. | n.a. | n.a. |
| 5th percentile | 0.17 | 0.18 | 0.35 | 0.13 | 0.66 | 0.70 | 0.45 | 0.24 | 0.23 |
| 95th percentile | 0.84 | 0.82 | 0.72 | 0.13 | 0.79 | 0.70 | 0.45 | 0.24 | 0.23 |
Plasma samples from participants at baseline were tested for their sensitivity to BLV in PHHs. Mean, median, range, and the 5th and 95th percentiles are shown.
BLV, bulevirtide; GT, genotype; n.a., not applicable owing to low infectivity; PHH, primary human hepatocyte.
Most clinical isolates tested were carrying HBV GTD envelopes (n = 108). Notably, the EC50 value of these isolates were within a narrow range with the 95th percentile of EC50 values being only approximately four-fold higher than the 5th percentile of EC50 values.
Taken together, these data demonstrate that BLV maintains effective inhibition of HDV infection across genotypes of HDV and HBV.
Analysis of the relationship between viral infectivity and sensitivity to BLV in clinical samples from participants with CHD
An analysis was performed with all available HDV clinical specimens, comparing their viral infectivity and in vitro sensitivity to BLV. Despite differences in infectivity, all clinical isolates remained susceptible to BLV with mean EC50 values that ranged from 0.20 to 0.73 nM (Fig. 4C and Table 3). Overall, there was no major effect of viral input on EC50 value determination, which allowed for testing of the majority of clinical samples regardless of viral load (128/141, 91%).
In conclusion, similar EC50 values for BLV were observed regardless of the HBV/HDV genotype combination of the sample tested.
Analysis of the relationship between HDAg and PreS1 sequence in BLV sensitivity in clinical samples from patients with CHD
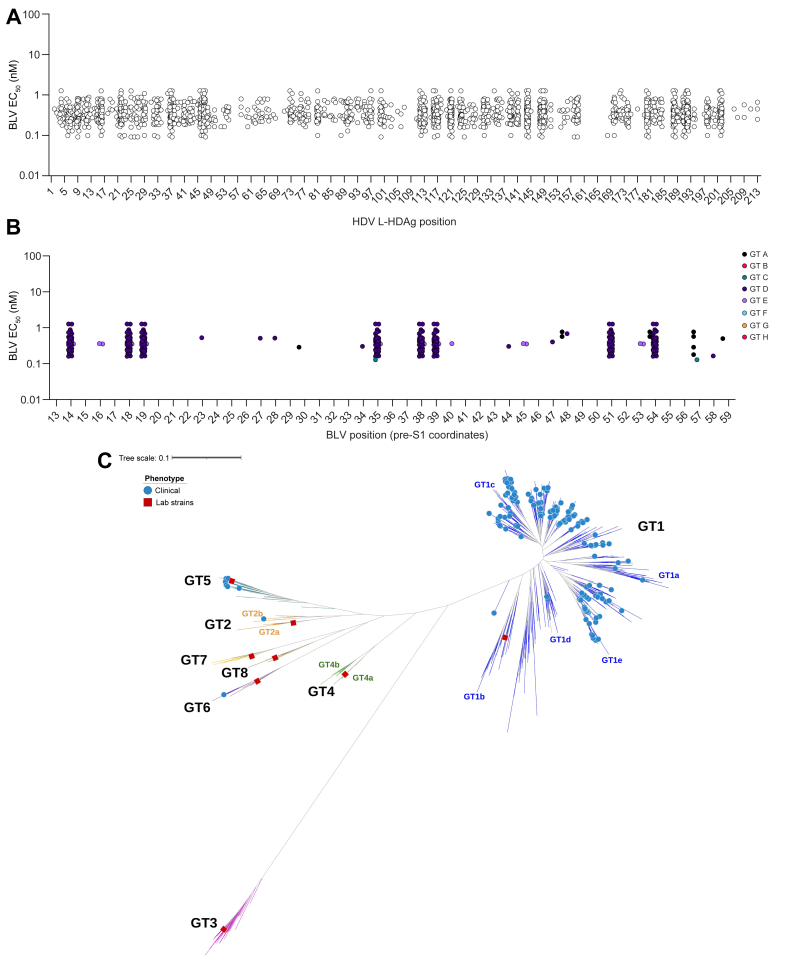
Next, a possible correlation between in vitro BLV activity and the sequence of the HDAg of all HDV clinical samples available was investigated. Because the majority of the clinical samples grouped to genotypes HBV-D/HDV-1, an HDV-1 consensus sequence from 10 reference sequences was constructed. Compared with this consensus, polymorphisms with several specific variations were identified. Despite the difference in sequence positions, the sensitivity to BLV remained comparable with EC50 values that ranged from 0.09 to 1.27 nM (Fig. 5A).
Fig. 5.
Effect of HDAg and HBV PreS1 polymorphisms on in vitro sensitivity to BLV.
(A) Median BLV EC50 values plotted against polymorphic positions in HADg from HDV-1. Each data point denotes a sample (clinical or laboratory strains) that contains a variant different from that of a consensus HDV-1 sequence derived from one representative sequence per HDV-1 subtype out of 10 reference sequences. (B) Median BLV EC50 values plotted against polymorphic positions of the BLV region of PreS1. Each data point represents a sample with a variant amino acid position different from the BLV sequence. (C) Phylogenetic tree of HDV genome highlighting all in vitro phenotyped laboratory strains (red squares) and clinical (blue circles) for their sensitivity to BLV. BLV, bulevirtide; GTA–H, genotypes A–H; HDAg, HDV antigen; HDV-1 to HDV-8, HDV genotypes 1–8.
Lastly, the sequence of the homologous BLV region to PreS1 (positions 13–59) of all clinical isolates was directly compared with the BLV sequence. This analysis revealed differences in positions at residues 14, 18, 19, 35, 38, 39, 51, and 54. Therefore, an analysis of the activity of BLV on isolates containing these variants was performed. As shown in Fig. 5, despite sequence differences, there was no significant effect on the sensitivity of these viral variants to inhibition with BLV in vitro, with EC50 values ranging from 0.13 to 1.27 nM (Fig. 5B).
Overall, despite the high sequence diversity of all the HDV clinical isolates and laboratory strains tested (Fig. 5C), all samples were sensitive to BLV in vitro with a very narrow EC50 range. This argues for the use of BLV as a therapy with potential broad-spectrum genotypic coverage in patients with CHD.
Discussion
This study describes the broad and potent activity of BLV in 81 recombinant laboratory strains, comprising HDV-1 to HDV-8 in combination with HBV L-HBsAg from HBV GTA–H, as well as a collection of 128 different clinical samples from patients with CHD.
A phenotyping assay using PHHs allowed us to determine the activity of BLV for HDV laboratory strains of genotypes 1, 2, 3, 5, 7, and 8 enveloped with L-HBsAg from HBV GTA–H. Similarly, HDV-4 combinations with HBV GTB (B/4), GTE (E/4), and GTG (G/4) and HDV-6 combinations with HBV GTA–E (A–E/6) and GTG (G/6) were analysed. All of these combinations were highly sensitive to BLV, with mean EC50 values that ranged from 0.34 to 0.71 nM. In addition, 24 HDV laboratory strains consisting of the HDV-1 genome enveloped with L-HBsAg containing the most common polymorphisms in HBV GTA–D (two for HBV GTA, 10 for HBV GTB, 10 for HBV GTC, and two for HBV GTD) representing >7,000 sequences from clinical isolates were generated. The corresponding HDVs were all sensitive to BLV with EC50 values <1 nM, indicating that the activity of BLV is not affected by the particular HBV PreS1 sequence in the envelope of the HDVs.
A conservation analysis of the PreS1 region performed after sequence alignment of the over 7,000 clinical isolates mentioned above revealed an overall high degree of conservation among all genotypes. In addition, the BLV sequence 9-NPGLFFP-15, crucial for binding to NTCP, was found to be extremely conserved among genotypes except for residue F25L in GTF (16.6% frequency; data not shown). Overall, polymorphisms in PreS1 were located far from the residues critical for NTCP binding and were not consistent across GTA–H. For GTA, only one residue was found to be variable in >5% of the sequences analysed, with GTC being the most different (five residues above the 5% cut-off value). GTB and GTD had two variable residues each. In summary, despite some PreS1 polymorphisms identified within genotypes, all viruses were sensitive to BLV treatment, highlighting the overall broad antiviral activity of BLV.
Similarly, a collection of 128 plasma samples from patients with CHD covering HBV/HDV genotype combinations D/1, D/2, A/1, A/5, A/6, C/1, B/1, E/1, and E/5 were blocked from entry into hepatocytes by BLV, with EC50 values that were within a narrow range of 0.1–1.3 nM, with the 95th percentile of EC50 values being only two- to five-fold higher than the 5th percentile of EC50 values. Sequence analysis of the BLV region of PreS1 in the envelope of these clinical isolates revealed 22 substitutions (22/47, 47%) when compared with the BLV sequence. These sequence variations did not significantly change inhibition by BLV. Although unlikely to affect sensitivity to BLV, 86 substitutions were identified in the HDAg of these clinical isolates and tested against BLV. As expected, they did not have an effect on sensitivity to BLV either (Fig. 5A).
For all clinical samples tested, the phenotyping assay showed a high correlation between the percentage of HDV-infected cells (percentage of HDAg-positive cells) and the patient’s viral load. More than half of the clinical samples tested (72/128, 56%) fell within the 0.1–5% range of HDAg-positive cells when using 2 μl of viral input. Clinical samples that fell in the highest >20% range (15/128, 12%) had BLV EC50 values that trended slightly higher than those with lower infectivity values although the differences were small (approximately two-fold). Regardless of differences in viral load/infectivity, all clinical samples tested were sensitive to BLV with mean EC50 values that ranged from 0.2 to 0.73 nM. Ninety-five percent (121/128) of the plasma samples tested were HDV-1, the most common and diverse HDV genotype. Karimzadeh et al.27 have identified four subgenotypes of HDV-1 with distinct geographic distribution: HDV-1a and HDV-1b in Sub-Saharan Africa; HDV-1c in the Western Pacific region; and HDV-1d, which is more common in Europe and Asia. Our internal phylogenetic analyses have revealed potential new HDV-1 subtypes based on distinct clusters of HDV-1 sequences from the same country.28 Most of these potential additional subtypes are represented in the clinical samples described here, and despite their sequence divergence (Fig. 5C), they remained highly susceptible to BLV with similar EC50 values.
Similar to the clinical samples, the BLV EC50 values against various HBV/HDV genotypes for the laboratory strains were within a narrow range from 0.17 to 0.93 nM, with the 95th percentile of EC50 values being only two- to three-fold higher than the 5th percentile of EC50 values. This <10-fold range in sensitivity to BLV described here is similar to that of sofosbuvir, a pan-genotypic nucleotide inhibitor approved for the treatment of HCV-infected patients, with an EC50 range of 0.014 to 0.11 μM for HCV-1 to HCV-6. Other approved HCV inhibitors, such as the NS5A protein-targeting velpatasvir, showed very potent viral inhibition but a much broader range (∼65-fold) of activity depending on the isolate, with EC50 values that ranged from 0.002 to 0.13 nM for HCV-1 to HCV-6.29 This argues for a favourable, broad-spectrum genotypic activity profile for BLV that resembles that of nucleotide inhibitors already available in the clinic for other hepatotropic viruses.
The analysis of the sensitivity of laboratory strains and clinical plasma samples to BLV described here was based on an infection assay using PHH. The percentage of HDAg-positive cells in the assay could be used as a proxy for the infectivity of the viruses tested. For laboratory strains, viruses were harvested on Day 8 after cotransfection and then used to infect PHH for 5 days using a constant viral inoculum. Therefore, the assay could reflect the kinetics of infectious HDV viral production for the various HBV/HDV genotype combinations. The infectivity of HDV genotypes, based on median infectivity values of all HBV/HDV combinations, ranked as follows: HDV-3 >HDV-6 ≈ HDV-8 >HDV-5 >HDV-1 >HDV-7 >HDV-2 >HDV-4 (Table S3). For HBV, the infectivity of the eight genotypes ranked as follows: GTE >GTB >GTG >GTD >GTA ≈ GTF >GTC >GTH (Table S3). Overall, most HDV genotypes, namely, HDV-3, HDV-6, HDV-8, HDV-5, and HDV-1, resulted in median infectivity values higher than 2%, whereas other genotypes resulted in very low infectivity, particularly HDV-4, with a median value of 0.05%. For HDV-4, combinations C/4 and D/4 had infectivity values <0.05%, whereas combinations A/4, F/4, and H/4 resulted in no detectable infectivity in the assay. Interestingly, for HDV-6, no infectivity was detected for combinations F/6 and H/6; however, all other combinations resulted in infectivity values that allowed for phenotyping against BLV. Consistent with other reports, our data show that the kinetics of formation of infectious HDV viral particles may vary depending on the particular HBV/HDV genotype combination.16 In fact, not all HDV genotypes were enveloped with the same efficiency by L-HBsAg envelopes from different HBV genotypes. Selective preference for envelopes of certain HBV genotypes in HDV genomic assembly has been described before. It was hypothesised that differences of assembly kinetics and/or de novo cell entry of some of the combinations may explain this phenomenon.16 It is likely that in addition to sensitivity to BLV, the virological response in patients will depend also on other factors, such as cell turnover, RNA decay, and the immune response. Although the sensitivity of all HDV/HBV genotypes to BLV in this single-round in vitro infection assay is very similar, the virological responses in patients infected with different genotypes and treated with BLV will warrant further investigation.30,31
This is the first report of BLV’s in vitro activity on a wide and representative collection of samples that include genotype combinations with very low infectivity that had hampered previous attempts at phenotyping. This was achieved through the establishment of a sensitive infectivity assay using PHHs. These promising results, with a large number of samples all susceptible to in vitro BLV inhibition, will have to be confirmed in vivo in patients with more prevalent HBV/HDV genotype combinations in circulation and rare HDV genotypes. The natural occurrence of HBV/HDV genotypes has been described in some studies: in China, HDV-1 was mainly associated with HBV GTC, although HDV-2 was also reported.32 In Taiwan, HDV-4 was the prevalent genotype (72%) circulating among injection drug users, whereas HDV-2 was predominant (73%) in the non-injection drug use populations.33 These HBV/HDV genotype combinations prevalent in China and Taiwan show similar in vitro BLV sensitivity compared to any other HBV/HDV genotype. However, clinical samples with these genotype combinations will need to be further investigated.
In summary, this study presents data on the potent activity of BLV on the most diverse collection of clinical samples from patients with CHD reported to date. Although the majority of the samples were HDV-1, some other less frequently found genotypes are described, such as HDV-2, -5, and -6. Overall, all isolates and laboratory strains displayed similar in vitro sensitivity to BLV, supporting its use as a novel therapy for patients with CHD, regardless of the particular combination of HBV/HDV genotypes they are infected with.
Financial support
Funding was received from Gilead Sciences.
Authors’ contributions
Writing of the manuscript: Rmat, HM. Data analysis: Rmat, HM. Interpretation of the data: Rmat, HM, SX, AS, TY, YL, LM, BH, DH, Rmar, SM, CR, CM, Tae, SC, DM, EM, HM, JH, SU, Tas, DA, PL. Experimental supervision: SX, AS, TY, YL, LM, BH, DH, Rmar, SM, CR, CM, Tae, SC, DM, EM, HM. Manuscript review: JH, SU, Tas, DA, PL. Sample supply: Tas, DA, PL. Manuscript drafting and review: all authors.
Data availability statement
The data of this study are available upon reasonable request.
Conflicts of interest
DA and JH report no conflicts of interest. RMat, YL, SX, SC, RM, SM, TAe, BH, TY, LM, DH, AS, RMar, DM, CR, EM, CM, and HM are employees and stockholders of Gilead Sciences. Tas and PL are consultants for Gilead Sciences. SU is a co-applicant and co-inventor on patents protecting Hepcludex (bulevirtide/Hepcludex) and consultant for Gilead Sciences. Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100893.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Sagnelli C., Sagnelli E., Russo A., Pisaturo M., Occhiello L., Coppola N. HBV/HDV co-infection: epidemiological and clinical changes, recent knowledge and future challenges. Life (Basel) 2021;11:169. doi: 10.3390/life11020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romeo R., Del Ninno E., Rumi M., Russo A., Sangiovanni A., de Franchis R., et al. A 28-year study of the course of hepatitis Δ infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology. 2009;136:1629–1638. doi: 10.1053/j.gastro.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 3.Stockdale A.J., Kreuels B., Henrion M.Y.R., Giorgi E., Kyomuhangi I., de Martel C., et al. The global prevalence of hepatitis D virus infection: systematic review and meta-analysis. J Hepatol. 2020;73:523–532. doi: 10.1016/j.jhep.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miao Z., Zhang S., Ou X., Li S., Ma Z., Wang W., et al. Estimating the global prevalence, disease progression, and clinical outcome of hepatitis Delta Virus Infection. J Infect Dis. 2020;221:1677–1687. doi: 10.1093/infdis/jiz633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papatheodoridi M., Papatheodoridis G.V. Is hepatitis delta underestimated? Liver Int. 2021;41(Suppl. 1):38–44. doi: 10.1111/liv.14833. [DOI] [PubMed] [Google Scholar]
- 6.Urban S., Neumann-Haefelin C., Lampertico P. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease. Gut. 2021;70:1782–1794. doi: 10.1136/gutjnl-2020-323888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalfi P., Kennedy P.T., Majzoub K., Asselah T. Hepatitis D virus: improving virological knowledge to develop new treatments. Antivir Res. 2023;209 doi: 10.1016/j.antiviral.2022.105461. [DOI] [PubMed] [Google Scholar]
- 8.Lempp F.A., Ni Y., Urban S. Hepatitis delta virus: insights into a peculiar pathogen and novel treatment options. Nat Rev Gastroenterol Hepatol. 2016;13:580–589. doi: 10.1038/nrgastro.2016.126. [DOI] [PubMed] [Google Scholar]
- 9.Rizzetto M. Hepatitis D: thirty years after. J Hepatol. 2009;50:1043–1050. doi: 10.1016/j.jhep.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Sureau C., Guerra B., Lanford R.E. Role of the large hepatitis B virus envelope protein in infectivity of the hepatitis delta virion. J Virol. 1993;67:366–372. doi: 10.1128/jvi.67.1.366-372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor J.M. Hepatitis D virus replication. Cold Spring Harb Perspect Med. 2015;5:a021568. doi: 10.1101/cshperspect.a021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wedemeyer H., Manns M.P. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol. 2010;7:31–40. doi: 10.1038/nrgastro.2009.205. [DOI] [PubMed] [Google Scholar]
- 13.Tu T., Urban S. Virus entry and its inhibition to prevent and treat hepatitis B and hepatitis D virus infections. Curr Opin Virol. 2018;30:68–79. doi: 10.1016/j.coviro.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Sunbul M. Hepatitis B virus genotypes: global distribution and clinical importance. World J Gastroenterol. 2014;20:5427–5434. doi: 10.3748/wjg.v20.i18.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang P.Y., Yang X., Guo L., Wang Y.W., Zhang W.L., Sun Y.X., et al. Establishment of a human cell line with a surface display system for screening and optimizing Na+-taurocholate cotransporting polypeptide-binding peptides. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.920280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W., Lempp F.A., Schlund F., Walter L., Decker C.C., Zhang Z., et al. Assembly and infection efficacy of hepatitis B virus surface protein exchanges in 8 hepatitis D virus genotype isolates. J Hepatol. 2021;75:311–323. doi: 10.1016/j.jhep.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Ni Y., Lempp F.A., Mehrle S., Nkongolo S., Kaufman C., Fälth M., et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Yan H., Zhong G., Xu G., He W., Jing Z., Gao Z., et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife. 2012;1 doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degasperi E., Anolli M.P., Lampertico P. Bulevirtide for patients with compensated chronic hepatitis delta: a review. Liver Int. 2023;(Suppl. 1):80–86. doi: 10.1111/liv.15389. [DOI] [PubMed] [Google Scholar]
- 20.Wedemeyer H., Schöneweis K., Bogomolov P., Blank A., Voronkova N., Stepanova T., et al. Safety and efficacy of bulevirtide in combination with tenofovir disoproxil fumarate in patients with hepatitis B virus and hepatitis D virus coinfection (MYR202): a multicentre, randomised, parallel-group, open-label, phase 2 trial. Lancet Infect Dis. 2023;23:117–129. doi: 10.1016/S1473-3099(22)00318-8. [DOI] [PubMed] [Google Scholar]
- 21.Herta T., Hahn M., Maier M., Fischer J., Niemeyer J., Honemann M., et al. Efficacy and safety of bulevirtide plus tenofovir disoproxil fumarate in real-world patients with chronic hepatitis B and D co-infection. Pathogens. 2022;11:517. doi: 10.3390/pathogens11050517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loglio A., Ferenci P., Uceda Renteria S.C., Tham C.Y.L., Scholtes C., Holzmann H., et al. Safety and effectiveness of up to 3 years' bulevirtide monotherapy in patients with HDV-related cirrhosis. J Hepatol. 2022;76:464–469. doi: 10.1016/j.jhep.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Asselah T., Loureiro D., Le Gal F., Narguet S., Brichler S., Bouton V., et al. Early virological response in six patients with hepatitis D virus infection and compensated cirrhosis treated with Bulevirtide in real-life. Liver Int. 2021;41:1509–1517. doi: 10.1111/liv.14950. [DOI] [PubMed] [Google Scholar]
- 24.Lampertico P., Roulot D., Wedemeyer H. Bulevirtide with or without pegIFNɑ for patients with compensated chronic hepatitis delta: from clinical trials to real-world studies. J Hepatol. 2022;77:1422–1430. doi: 10.1016/j.jhep.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Parallel Sessions. J Hepatol. 2021;75(Suppl. 2):S205–S293. [Google Scholar]
- 26.Oral Presentations. J Hepatol. 2022;77(Suppl. 1):S1–S18. [Google Scholar]
- 27.Karimzadeh H., Usman Z., Frishman D., Roggendorf M. Genetic diversity of hepatitis D virus genotype-1 in Europe allows classification into subtypes. J Viral Hepat. 2019;26:900–910. doi: 10.1111/jvh.13086. [DOI] [PubMed] [Google Scholar]
- 28.Manhas S, Xu S, Chang S, Aeschbacher T, Mateo R, Martin R, et al. HDV full genome sequencing and sensitive HBV genotyping from a large cohort of HBV/HDV co-infected patients. EASL 2023 Poster.
- 29.Cory T.J., Mu Y., Gong Y., Kodidela S., Kumar S. Sofosbuvir + velpatasvir + voxilaprevir for the treatment of hepatitis C infection. Expert Opin Pharmacother. 2018;19:749–757. doi: 10.1080/14656566.2018.1459567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sureau C., Negro F. The hepatitis delta virus: replication and pathogenesis. J Hepatol. 2016;64:S102–S116. doi: 10.1016/j.jhep.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Usman Z., Velkov S., Protzer U., Roggendorf M., Frishman D., Karimzadeh H. HDVdb: a comprehensive hepatitis D virus database. Viruses. 2020;12:538. doi: 10.3390/v12050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu S., Zhang Y., Tang Y., Yao T., Lv M., Tang Z., et al. Molecular epidemiology and clinical characteristics of hepatitis delta virus (HDV) infected patients with elevated transaminases in Shanghai, China. BMC Infect Dis. 2020;20:565. doi: 10.1186/s12879-020-05275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin H.H., Lee S.S., Yu M.L., Chang T.T., Su C.W., Hu B.S., et al. Changing hepatitis D virus epidemiology in a hepatitis B virus endemic area with a national vaccination program. Hepatology. 2015;61:1870–1879. doi: 10.1002/hep.27742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study are available upon reasonable request.