Abstract
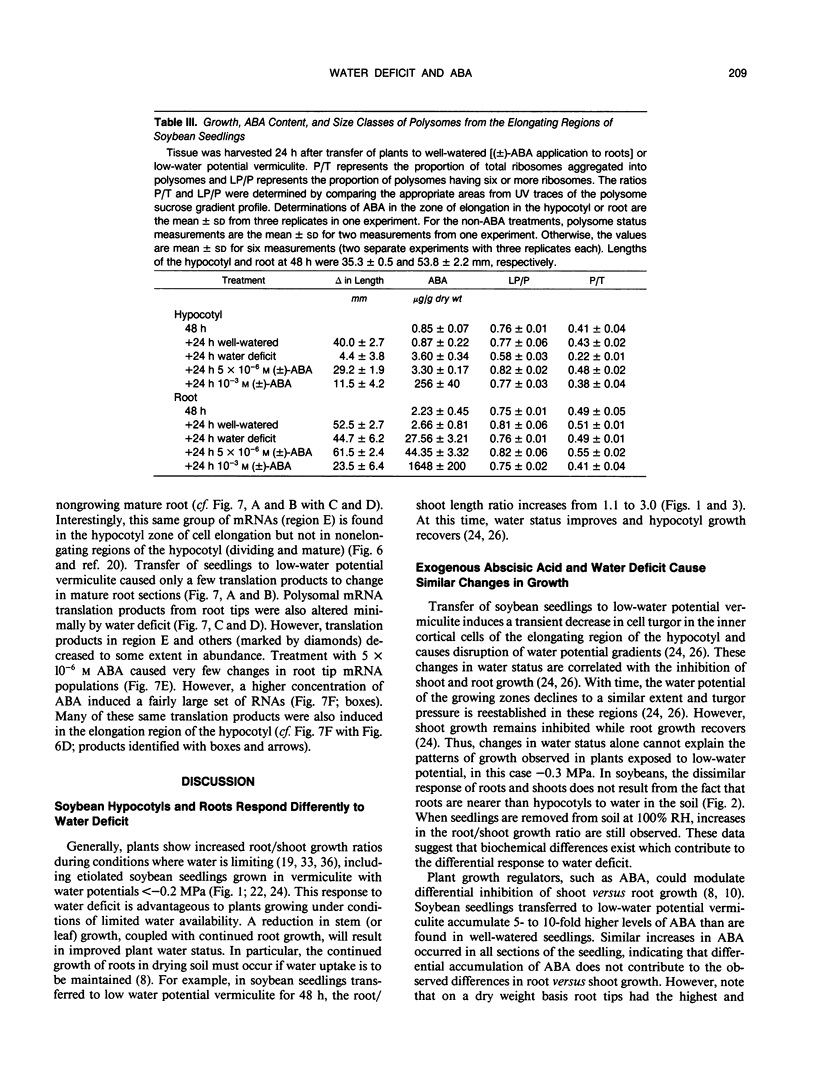
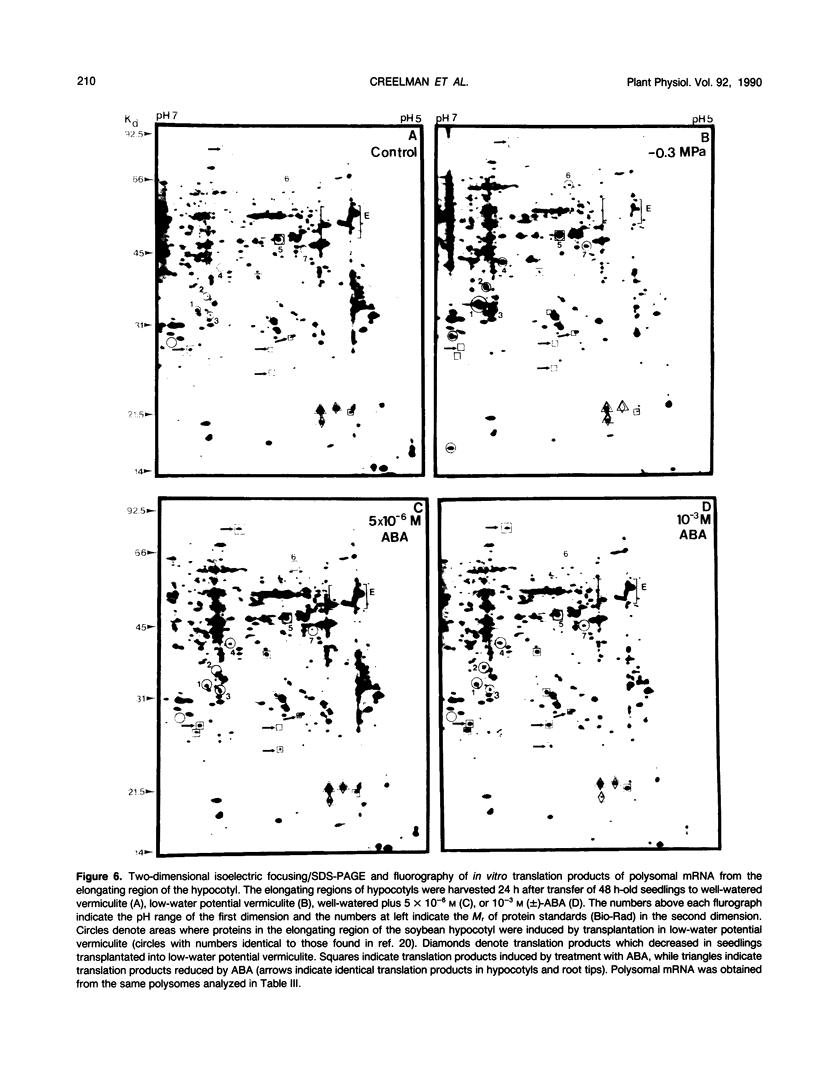
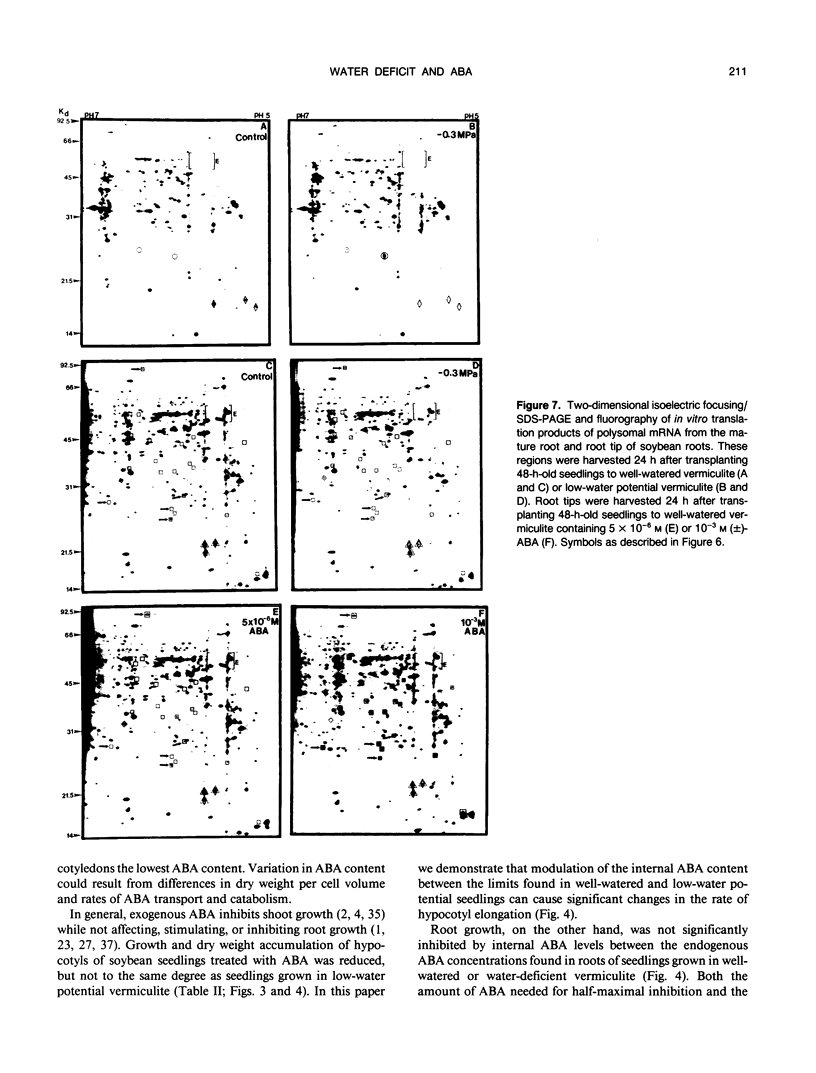
Roots often continue to elongate while shoot growth is inhibited in plants subjected to low-water potentials. The cause of this differential response to water deficit was investigated. We examined hypocotyl and root growth, polysome status and mRNA populations, and abscisic acid (ABA) content in etiolated soybean (Glycine max [L.] Merr. cv Williams) seedlings whose growth was inhibited by transfer to low-water potential vermiculite or exogenous ABA. Both treatments affected growth and dry weight in a similar fashion. Maximum inhibition of hypocotyl growth occurred when internal ABA levels (modulated by ABA application) reached the endogenous level found in the elongating zone of seedlings grown in water-deficient vermiculite. Conversely, root growth was affected to only a slight extent in low-water potential seedlings and by most ABA treatments (in some, growth was promoted). In every seedling section examined, transfer of seedlings into low-water potential vermiculite caused ABA levels to increase approximately 5- to 10-fold over that found in well-watered seedlings. Changes in soluble sugar content, polysome status, and polysome mRNA translation products seen in low-water potential seedlings did not occur with ABA treatments sufficient to cause significant inhibition of hypocotyl elongation. These data suggest that both variation in endogenous ABA levels, and differing sensitivity to ABA in hypocotyls and roots can modulate root/shoot growth ratios. However, exogenous ABA did not induce changes in sugar accumulation, polysome status, and mRNA populations seen after transfer into low-water potential vermiculite.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensen R. J., Boyer J. S., Mullet J. E. Water deficit-induced changes in abscisic Acid, growth, polysomes, and translatable RNA in soybean hypocotyls. Plant Physiol. 1988 Oct;88(2):289–294. doi: 10.1104/pp.88.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray E. A. Drought- and ABA-Induced Changes in Polypeptide and mRNA Accumulation in Tomato Leaves. Plant Physiol. 1988 Dec;88(4):1210–1214. doi: 10.1104/pp.88.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri A. J., Boyer J. S. Water potentials induced by growth in soybean hypocotyls. Plant Physiol. 1982 Feb;69(2):492–496. doi: 10.1104/pp.69.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero F. D., Mullet J. E. Reduction of turgor induces rapid changes in leaf translatable RNA. Plant Physiol. 1988 Oct;88(2):401–408. doi: 10.1104/pp.88.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila J. J., Papp J. E., Schultz G. A., Bewley J. D. Induction of heat shock protein messenger RNA in maize mesocotyls by water stress, abscisic Acid, and wounding. Plant Physiol. 1984 Sep;76(1):270–274. doi: 10.1104/pp.76.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao T. C. Rapid Changes in Levels of Polyribosomes in Zea mays in Response to Water Stress. Plant Physiol. 1970 Aug;46(2):281–285. doi: 10.1104/pp.46.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason H. S., Mullet J. E., Boyer J. S. Polysomes, Messenger RNA, and Growth in Soybean Stems during Development and Water Deficit. Plant Physiol. 1988 Mar;86(3):725–733. doi: 10.1104/pp.86.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Riazi A. Stress-induced osmotic adjustment in growing regions of barley leaves. Plant Physiol. 1981 Sep;68(3):571–576. doi: 10.1104/pp.68.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H., Boyer J. S., Steudle E. Pressure probe and isopiestic psychrometer measure similar turgor. Plant Physiol. 1987 Mar;83(3):592–595. doi: 10.1104/pp.83.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H., Boyer J. S. Turgor and growth at low water potentials. Plant Physiol. 1989 Mar;89(3):798–804. doi: 10.1104/pp.89.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm M. M., Cline M. G. Rapid growth inhibition of Avena coleoptile segments by abscisic Acid. Plant Physiol. 1973 Jan;51(1):93–96. doi: 10.1104/pp.51.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P., Saugy M., Pilet P. E. Quantification of abscisic Acid in a single maize root. Plant Physiol. 1987 Sep;85(1):8–9. doi: 10.1104/pp.85.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Hormonal regulation of protein synthesis associated with salt tolerance in plant cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):739–743. doi: 10.1073/pnas.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]