Abstract
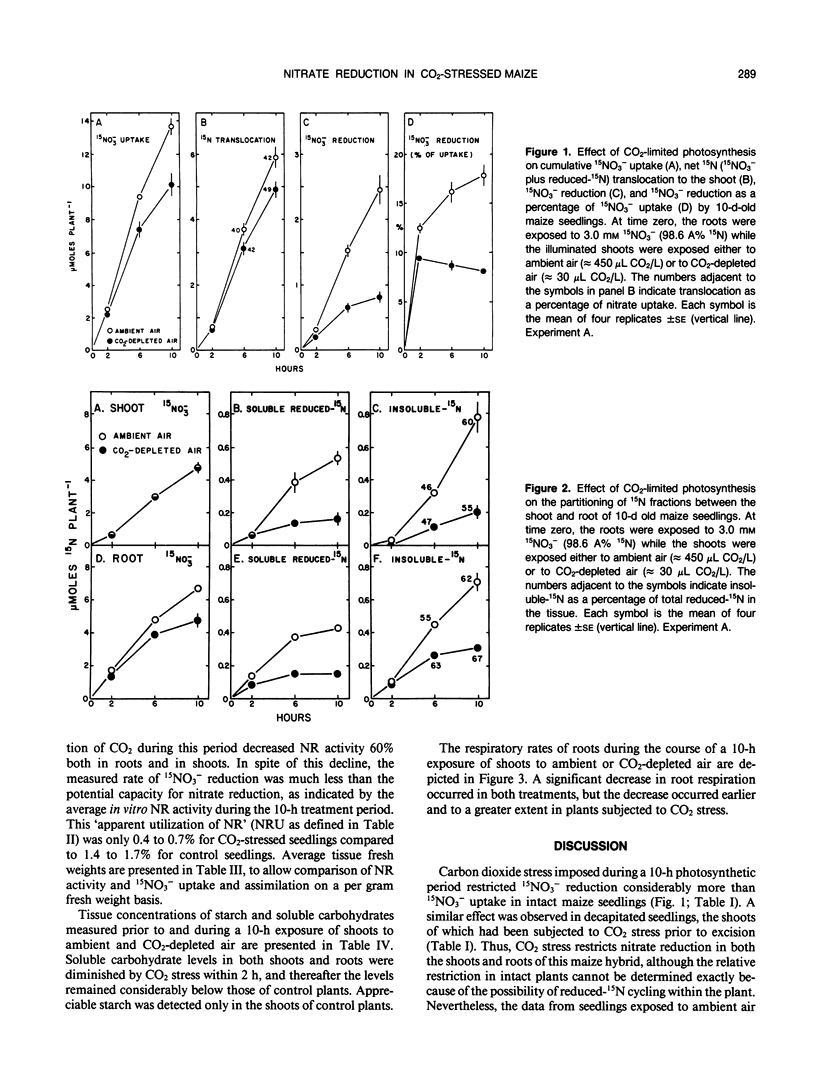
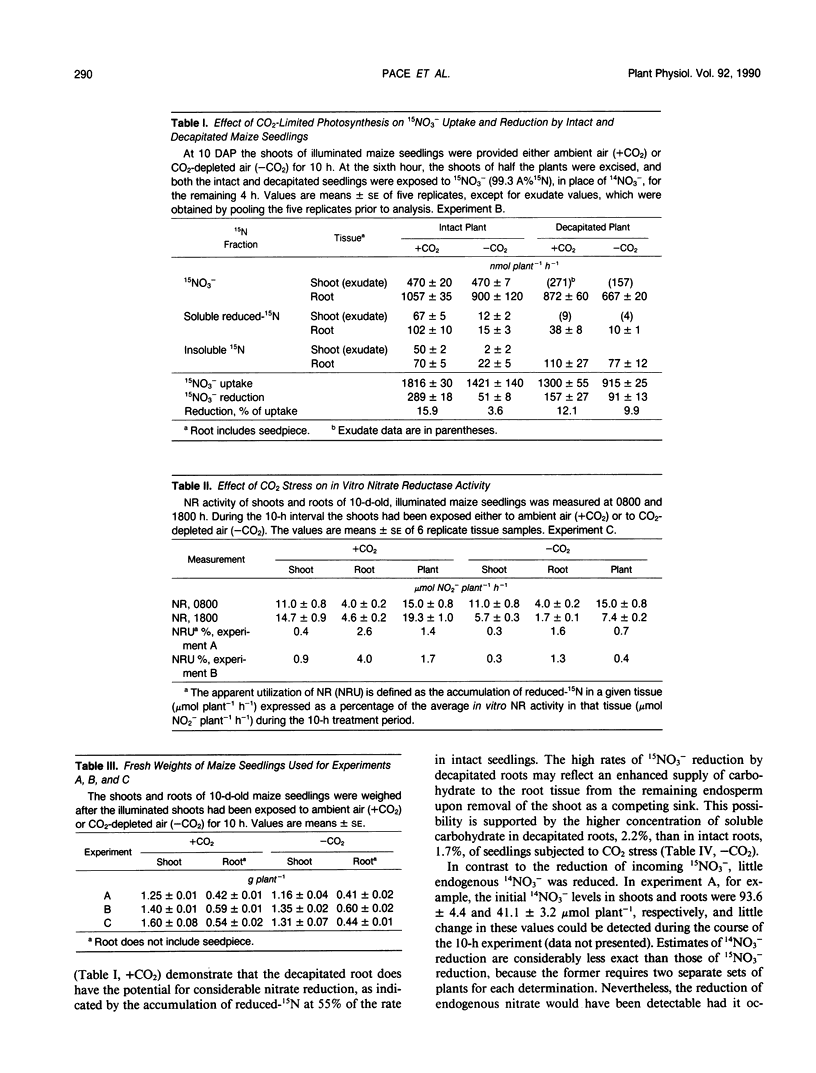
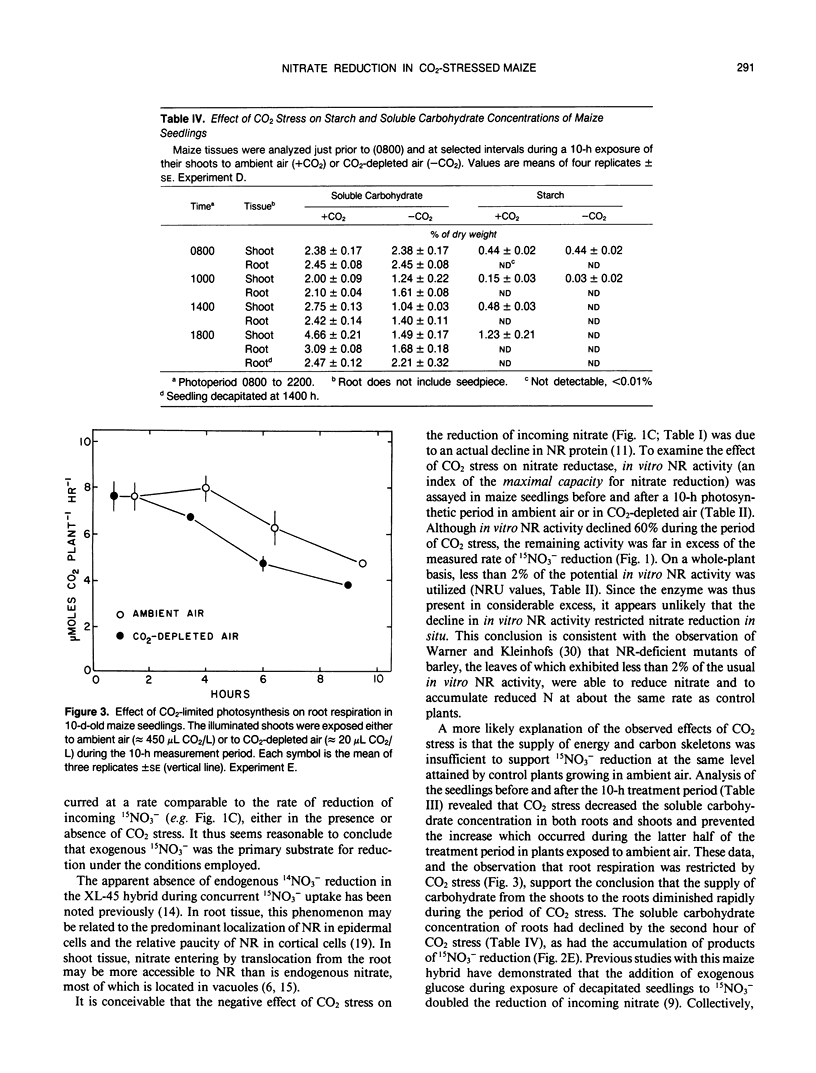
The effects of CO2-limited photosynthesis on 15NO3− uptake and reduction by maize (Zea mays, DeKalb XL-45) seedlings were examined in relation to concurrent effects of CO2 stress on carbohydrate levels and in vitro nitrate reductase activities. During a 10-hour period in CO2-depleted air (30 microliters of CO2/ per liter), cumulative 15NO3− uptake and reduction were restricted 22 and 82%, respectively, relative to control seedlings exposed to ambient air containing 450 microliters of CO2 per liter. The comparable values for roots of decapitated maize seedlings, the shoots of which had previously been subjected to CO2 stress, were 30 and 42%. The results demonstrate that reduction of entering nitrate by roots as well as shoots was regulated by concurrent photosynthesis. Although in vitro nitrate reductase activity of both tissues declined by 60% during a 10-hour period of CO2 stress, the remaining activity was greatly in excess of that required to catalyze the measured rate of 15NO3− reduction. Root respiration and soluble carbohydrate levels in root tissue were also decreased by CO2 stress. Collectively, the results indicate that nitrate uptake and reduction were regulated by the supply of energy and carbon skeletons required to support these processes, rather than by the potential enzymatic capacity to catalyze nitrate reduction, as measured by in vitro nitrate reductase activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Huffaker R. C. Dependency of Nitrate Reduction on Soluble Carbohydrates in Primary Leaves of Barley under Aerobic Conditions. Plant Physiol. 1984 Jul;75(3):623–628. doi: 10.1104/pp.75.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Huffaker R. C., Rains D. W., Rao K. P. Influence of light and ambient carbon dioxide concentration on nitrate assimilation by intact barley seedlings. Plant Physiol. 1979 Jun;63(6):1205–1209. doi: 10.1104/pp.63.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Rosichan J. L., Huffaker R. C. Comparative induction of nitrate reductase by nitrate and nitrite in barley leaves. Plant Physiol. 1987;83:579–584. doi: 10.1104/pp.83.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti N., Hageman R. H. Comparison of in Vivo and in Vitro Assays of Nitrate Reductase in Wheat (Triticum aestivum L.) Seedlings. Plant Physiol. 1976 Oct;58(4):583–587. doi: 10.1104/pp.58.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg M. D., Sullivan C. Y., Eastin J. D. A sensitive technique for the rapid measurement of carbon dioxide concentrations. Plant Physiol. 1978 Dec;62(6):924–926. doi: 10.1104/pp.62.6.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granstedt R. C., Huffaker R. C. Identification of the leaf vacuole as a major nitrate storage pool. Plant Physiol. 1982 Aug;70(2):410–413. doi: 10.1104/pp.70.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper L., Flesher D., Hageman R. H. Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol. 1971 Nov;48(5):580–590. doi: 10.1104/pp.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks A., Aslam M., Boesel I. Ammonium and amino acids as regulators of nitrate reductase in corn roots. Plant Physiol. 1977 Mar;59(3):391–394. doi: 10.1104/pp.59.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace G. M., Mackown C. T., Volk R. J. Minimizing Nitrate Reduction during Kjeldahl Digestion of Plant Tissue Extracts and Stem Exudates : APPLICATION TO N STUDIES. Plant Physiol. 1982 Jan;69(1):32–36. doi: 10.1104/pp.69.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis A. C., Peters D. B., Hageman R. H. Effect of carbon dioxide on nitrate accumulation and nitrate reductase induction in corn seedlings. Plant Physiol. 1974 Jun;53(6):934–941. doi: 10.1104/pp.53.6.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Thomas J. F., Remmler J. L., Campbell W. H., Volk R. J. Intercellular localization of nitrate reductase in roots. Plant Physiol. 1986 Nov;82(3):675–680. doi: 10.1104/pp.82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney S. K., Naik M. S. Role of light in the synthesis of nitrate reductase and nitrite reductase in rice seedlings. Biochem J. 1972 Nov;130(2):475–485. doi: 10.1042/bj1300475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Huffaker R. C. Light-induced Development of Polyribosomes and the Induction of Nitrate Reductase in Corn Leaves. Plant Physiol. 1970 Dec;46(6):800–805. doi: 10.1104/pp.46.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk R. J., Pearson C. J., Jackson W. A. Reduction of plant tissue nitrate to nitric oxide for mass spectrometric 15N analysis. Anal Biochem. 1979 Aug;97(1):131–135. doi: 10.1016/0003-2697(79)90336-1. [DOI] [PubMed] [Google Scholar]
- Warner R. L. Nitrate Utilization by Nitrate Reductase-deficient Barley Mutants. Plant Physiol. 1981 Apr;67(4):740–743. doi: 10.1104/pp.67.4.740. [DOI] [PMC free article] [PubMed] [Google Scholar]


