Abstract
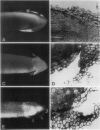
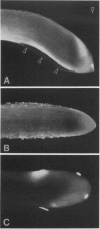
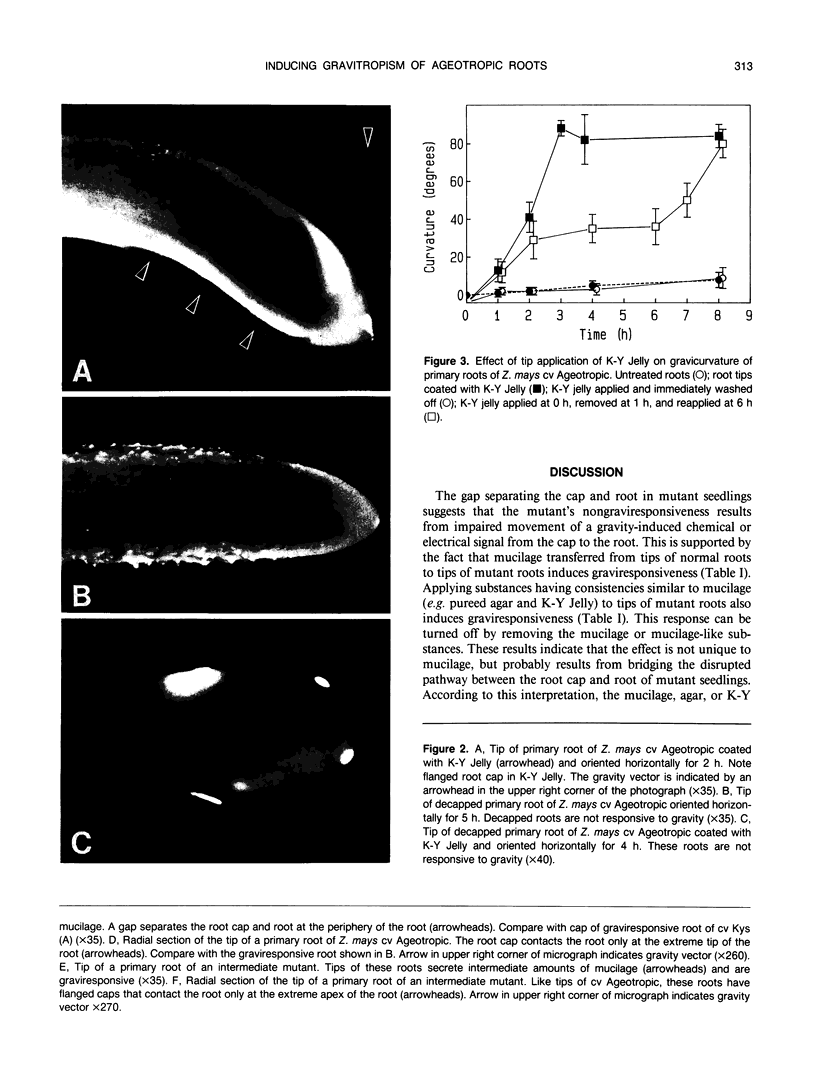
Primary roots of the mutant `Ageotropic' cultivar of Zea mays are nonresponsive to gravity. Their root caps secrete little or no mucilage and touch the root only at the extreme apex. A gap separates the cap and root at the periphery of the cap. Applying mucilage from normal roots or substances with a consistency similar to that of mucilage to tips of mutant roots causes these roots to become strongly graviresponsive. Gravicurvature stops when these substances are removed. Caps of some mutants secrete small amounts of mucilage and are graviresponsive. These results indicate that (a) the lack of graviresponsiveness in the mutant results from disrupting the transport pathway between the cap and root, (b) movement of the growth-modifying signal from the cap to the root occurs via an apoplastic pathway, and (c) mucilage is necessary for normal communication between the root cap and root in Zea mays cv Ageotropic.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caspar T., Pickard B. G. Gravitropism in a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity sensing. Planta. 1989;177:185–197. [PubMed] [Google Scholar]
- Evans M. L., Moore R., Hasenstein K. H. How roots respond to gravity. Sci Am. 1986 Dec;255(6):112–119. doi: 10.1038/scientificamerican1286-112. [DOI] [PubMed] [Google Scholar]
- Feldman L. J. Root gravitropism. Physiol Plant. 1985;65:341–344. doi: 10.1111/j.1399-3054.1985.tb02405.x. [DOI] [PubMed] [Google Scholar]
- Gibbons G. S., Wilkins M. B. Growth inhibitor production by root caps in relation to geotropic responses. Nature. 1970 May 9;226(5245):558–559. doi: 10.1038/226558a0. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Mulkey T. J., Evans M. L. Gravity-Induced Polar Transport of Calcium across Root Tips of Maize. Plant Physiol. 1983 Dec;73(4):874–876. doi: 10.1104/pp.73.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Mulkey T. J., Evans M. L. Inhibition of polar calcium movement and gravitropism in roots treated with auxin-transport inhibitors. Planta. 1984;160:536–543. [PubMed] [Google Scholar]
- Lee J. S., Mulkey T. J., Evans M. L. Reversible loss of gravitropic sensitivity in maize roots after tip application of calcium chelators. Science. 1983 Jun 24;220(4604):1375–1376. doi: 10.1126/science.220.4604.1375. [DOI] [PubMed] [Google Scholar]
- Moore R. A morphometric analysis of the redistribution of organelles in columella cells in primary roots of normal seedlings and agravitropic mutants of Hordeum vulgare. J Exp Bot. 1985 Aug;36(169):1275–1286. doi: 10.1093/jxb/36.8.1275. [DOI] [PubMed] [Google Scholar]
- Moore R., Cameron I. L., Hunter K. E., Olmos D., Smith N. K. The locations and amounts of endogenous ions and elements in the cap and elongating zone of horizontally oriented roots of Zea mays L.: an electron-probe EDS study. Ann Bot. 1987;59:667–677. doi: 10.1093/oxfordjournals.aob.a087365. [DOI] [PubMed] [Google Scholar]
- Moore R. Cytochemical localization of calcium in cap cells of primary roots of Zea mays L. J Exp Bot. 1986 Jan;37(174):73–79. doi: 10.1093/jxb/37.1.73. [DOI] [PubMed] [Google Scholar]
- Moore R., Evans M. L. How roots perceive and respond to gravity. Am J Bot. 1986 Apr;73(4):574–587. [PubMed] [Google Scholar]
- Moore R., Fondren W. M. A gradient of endogenous calcium forms in mucilage of graviresponding roots of Zea mays. Ann Bot. 1988;61:113–116. doi: 10.1093/oxfordjournals.aob.a087523. [DOI] [PubMed] [Google Scholar]
- Moore R., Fondren W. M., Marcum H. Characterization of root agravitropism induced by genetic, chemical, and developmental constraints. Am J Bot. 1987 Mar;74(3):329–336. [PubMed] [Google Scholar]
- Moore R., Fondren W. M. The possible involvement of root-cap mucilage in gravitropism and calcium movement across root tips of Allium cepa L. Ann Bot. 1986;58:381–387. doi: 10.1093/oxfordjournals.aob.a087216. [DOI] [PubMed] [Google Scholar]
- Moore R., McClelen C. E. Characterizing pathways by which gravitropic effectors could move from the root cap to the root of primary roots of Zea mays. Ann Bot. 1989;64:415–423. doi: 10.1093/oxfordjournals.aob.a087860. [DOI] [PubMed] [Google Scholar]
- Moore R. Root graviresponsiveness and cellular differentiation in wild-type and a starchless mutant of Arabidopsis thaliana. Ann Bot. 1989;64:271–277. doi: 10.1093/oxfordjournals.aob.a087841. [DOI] [PubMed] [Google Scholar]
- Moore R., Smith J. D. Graviresponsiveness and abscisic-acid content of roots of carotenoid-deficient mutants of Zea mays L. Planta. 1985;164:126–128. [PubMed] [Google Scholar]
- Ramsay A. T. Occurrence of biogenic siliceous sediments in the Atlantic ocean. Nature. 1971 Sep 10;233(5315):115–117. doi: 10.1038/233115a0. [DOI] [PubMed] [Google Scholar]