Abstract
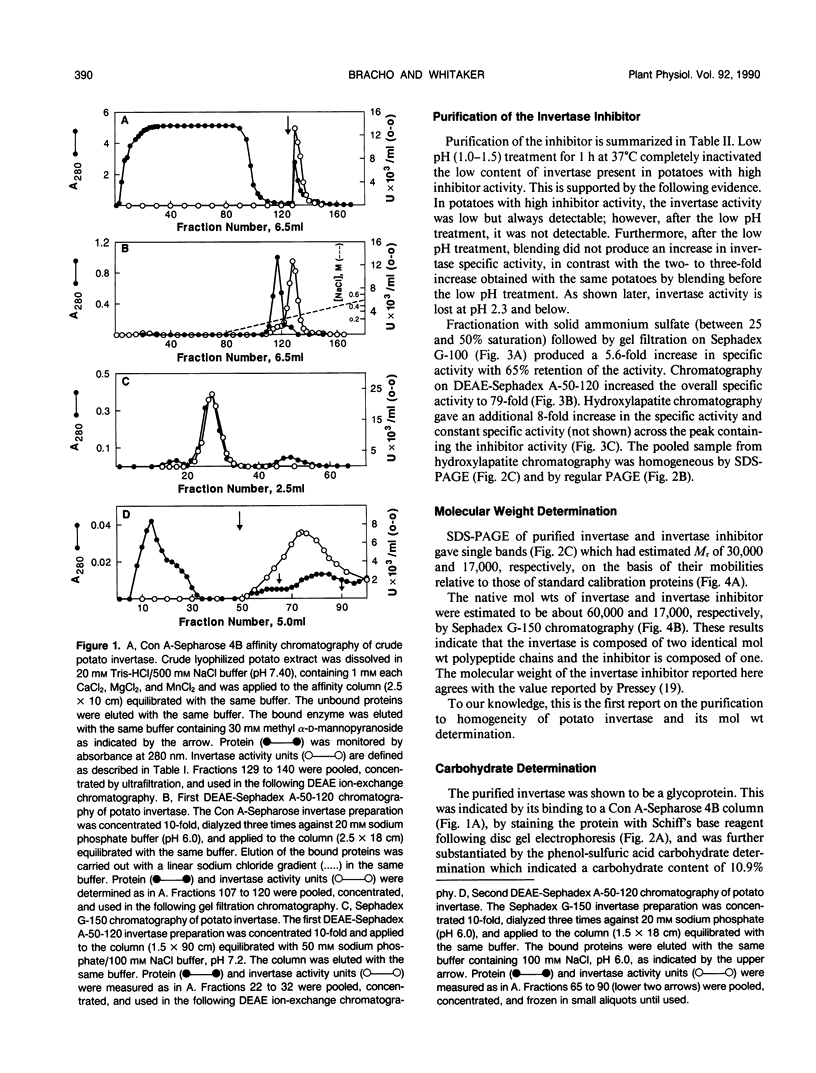
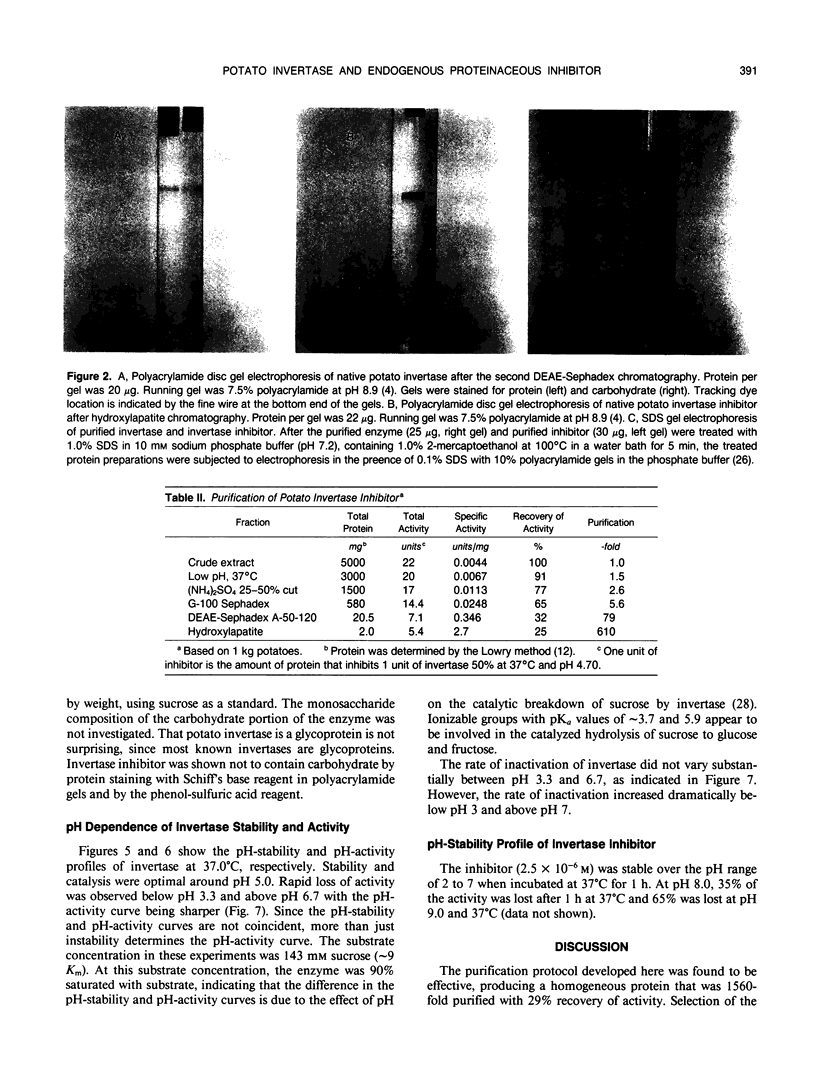
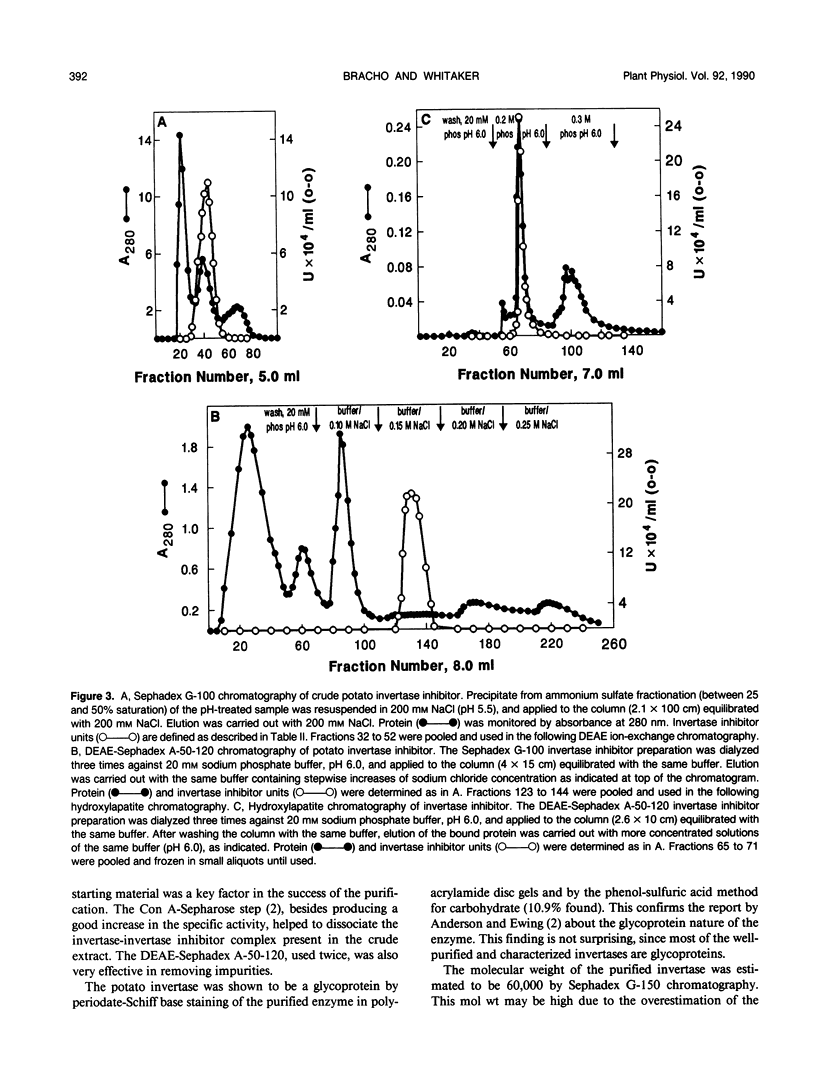
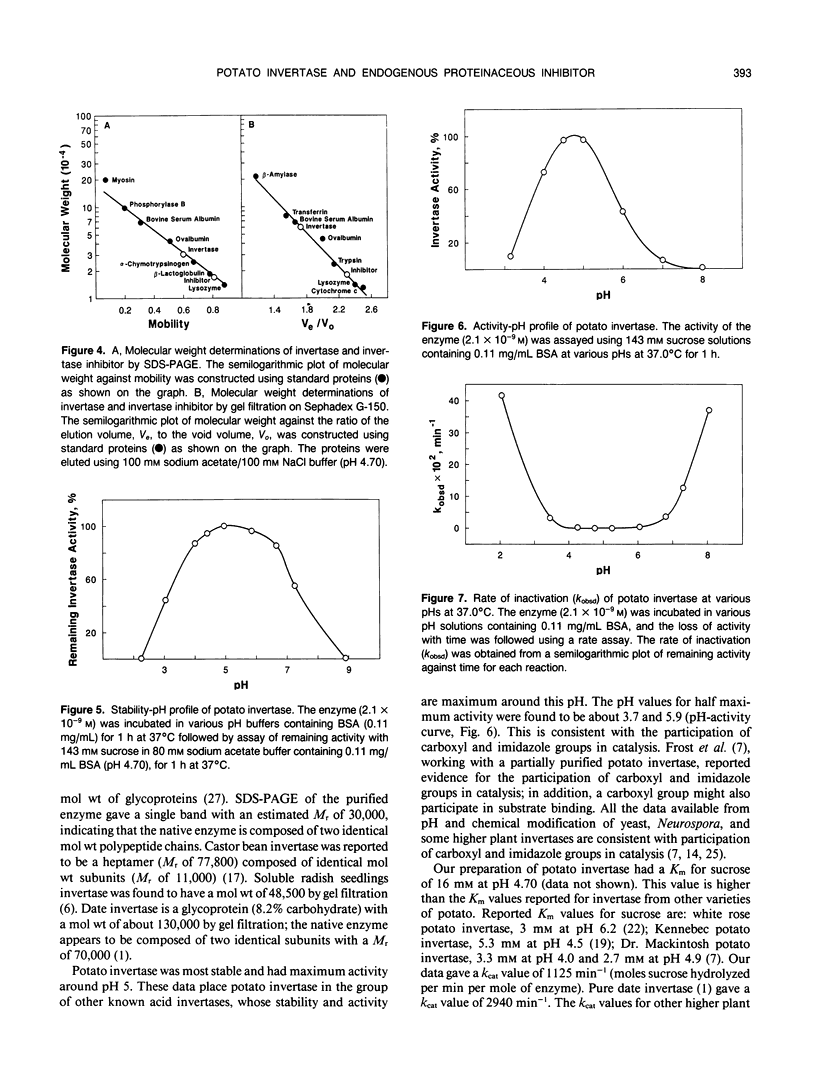
Invertase plays an important role in the hydrolysis of sucrose in higher plants, especially in the storage organs. In potato (Solanum tuberosum) tubers, and in some other plant tissues, the enzyme seems to be controlled by interaction with an endogenous proteinaceous inhibitor. An acid invertase from potato tubers (variety russet) was purified 1560-fold to electrophoretic homogeneity by consecutive use of concanvalin A-Sepharose 4B affinity chromatography, DEAE-Sephadex A-50-120 chromatography, Sephadex G-150 chromatography, and DEAE-Sephadex A-50-120 chromatography. The enzyme contained 10.9% carbohydrate, had an apparent molecular weight of 60,000 by gel filtration, and was composed of two identical molecular weight subunits (Mr 30,000). The enzyme had a Km for sucrose of 16 millimolar at pH 4.70 and was most stable and had maximum activity around pH 5. The endogenous inhibitor was purified 610-fold to homogeneity by consecutive treatment at pH 1 to 1.5 at 37°C for 1 hour, (NH4)2SO4 fractionation, Sephadex G-100 chromatography, DEAE-Sephadex G-50-120 chromatography, and hydroxylapatite chromatography. The inhibitor appears to be a single polypeptide (Mr 17,000) without glyco groups. The purified inhibitor was stable over the pH range of 2 to 7 when incubated at 37°C for 1 hour.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Frost G. M., Greenshields R. N., Teale F. W. Some properties of beta-fructofuranosidases partially purified from Phaseolus vulgaris and Solanum tuberosum. Biochem J. 1968 May;107(5):625–636. doi: 10.1042/bj1070625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON M. J. A rapid micromethod for estimation of non-volatile organic matter. J Biol Chem. 1949 Dec;181(2):707–711. [PubMed] [Google Scholar]
- Krishnan H. B., Blanchette J. T., Okita T. W. Wheat invertases : characterization of cell wall-bound and soluble forms. Plant Physiol. 1985 Jun;78(2):241–245. doi: 10.1104/pp.78.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- METZENBERG R. L. The purification and properties of invertase of Neurospora. Arch Biochem Biophys. 1963 Mar;100:503–511. doi: 10.1016/0003-9861(63)90118-8. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Uritani I. Isolation and characterization of acid invertase inhibitor from sweet potato. J Biochem. 1976 Mar;79(3):633–639. doi: 10.1093/oxfordjournals.jbchem.a131107. [DOI] [PubMed] [Google Scholar]
- Neumann N. P., Lampen J. O. Purification and properties of yeast invertase. Biochemistry. 1967 Feb;6(2):468–475. doi: 10.1021/bi00854a015. [DOI] [PubMed] [Google Scholar]
- Prado F. E., Vattuone M. A., Fleischmacher O. L., Sampietro A. R. Purification and characterization of Ricinus communis invertase. J Biol Chem. 1985 Apr 25;260(8):4952–4957. [PubMed] [Google Scholar]
- Pressey R. Invertase inhibitor from potatoes: purification, characterization, and reactivity with plant invertases. Plant Physiol. 1967 Dec;42(12):1780–1786. doi: 10.1104/pp.42.12.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. Invertase inhibitors from red beet, sugar beet, and sweet potato roots. Plant Physiol. 1968 Sep;43(9):1430–1434. doi: 10.1104/pp.43.9.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. Separation and properties of potato invertase and invertase inhibitor. Arch Biochem Biophys. 1966 Mar;113(3):667–674. doi: 10.1016/0003-9861(66)90246-3. [DOI] [PubMed] [Google Scholar]
- Schwimmer S., Makower R. U., Rorem E. S. Invertase & invertase inhibitor in potato. Plant Physiol. 1961 May;36(3):313–316. doi: 10.1104/pp.36.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. B., Knox R. B. Invertases of Lilium Pollen : Characterization and Activity during In Vitro Germination. Plant Physiol. 1984 Mar;74(3):510–515. doi: 10.1104/pp.74.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A., Shall S. Chemical modification of the active site of yeast invertase. Biochim Biophys Acta. 1971 Jul 21;242(1):172–189. doi: 10.1016/0005-2744(71)90097-0. [DOI] [PubMed] [Google Scholar]



