Abstract
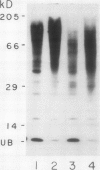
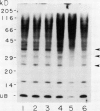
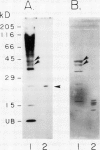
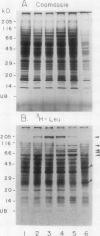
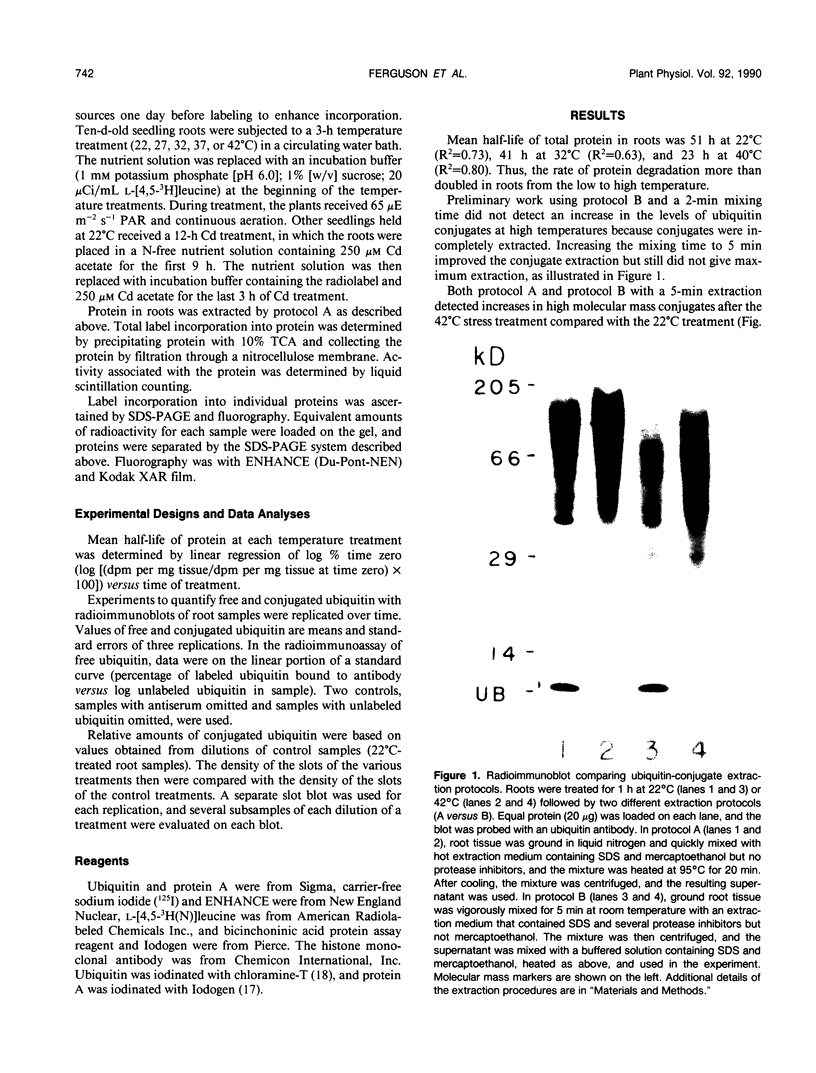
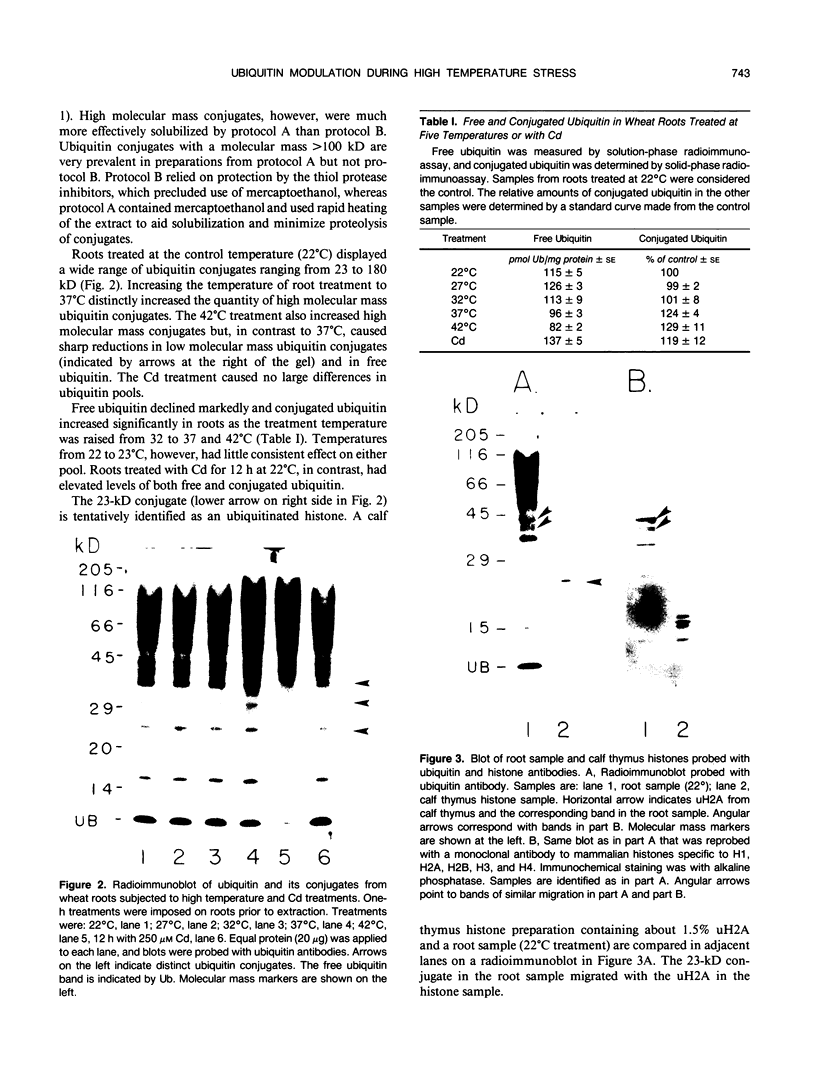
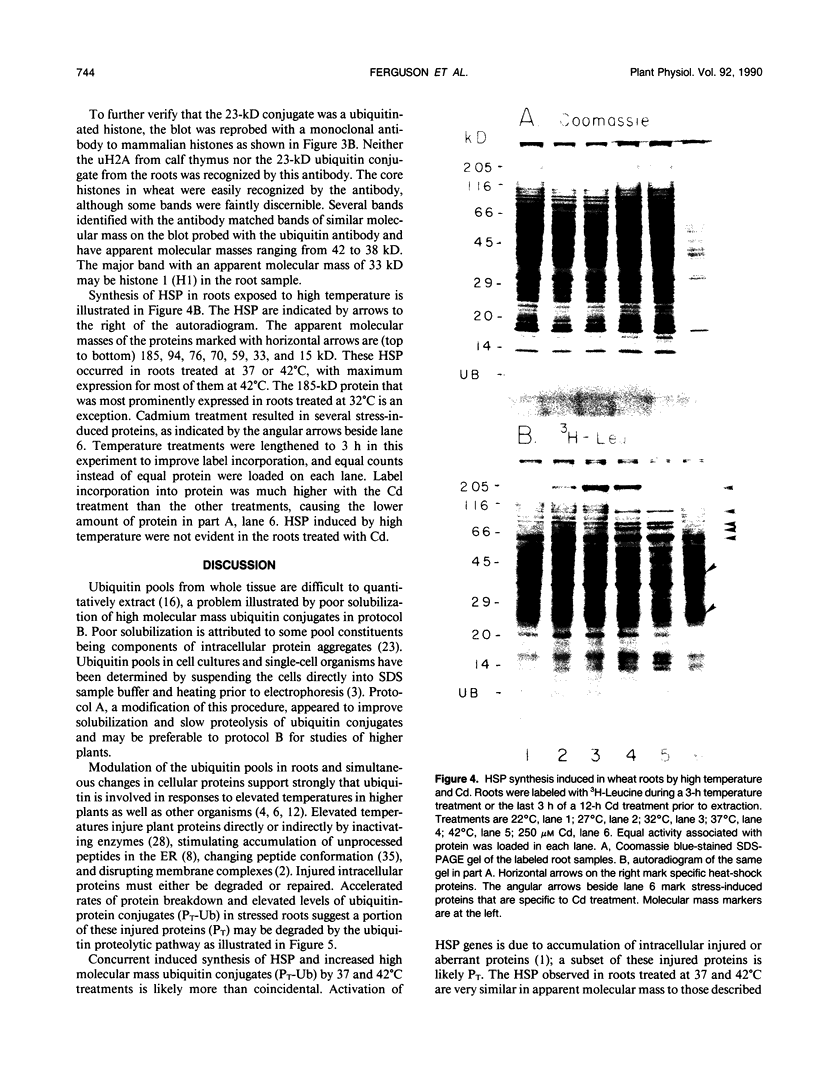
Ubiquitin, a key component in an ATP-dependent proteolytic pathway, participates in the response of various eucaryotic organisms to high temperature stress. Our objective was to determine if ubiquitin serves a similar capacity for metabolizing altered proteins in higher plants during stress. Degradation of total proteins was measured, and ubiquitin pools (free versus conjugated) were extracted with an improved protocol from wheat (Triticum aestivum L. cv Len) roots treated at 22, 27, 32, 37, and 42°C for 1 hour and assayed by western blots and radioimmunoassays. Heat-shock protein synthesis was detected by in vivo labeling and autoradiography. Mean half-life of total root proteins decreased from 51 hours at 22°C to 23 hours at 40°C. Ubiquitin pools were extracted better and proteolysis was slowed more by the improved protocol than by a conventional procedure for plant proteins. Amounts of high molecular mass conjugates were elevated and levels of low molecular mass conjugates and free ubiquitin were depressed when roots were treated at 37 or 42°C than at lower temperatures; the same high temperatures also induced synthesis of heat-shock proteins. We concluded that high temperatures increase breakdown of root proteins, which are degraded via the ubiquitin proteolytic pathway. A conjugate with an apparent molecular mass of 23 kilodaltons was tentatively identified as an ubiquitinated histone.
Full text
PDF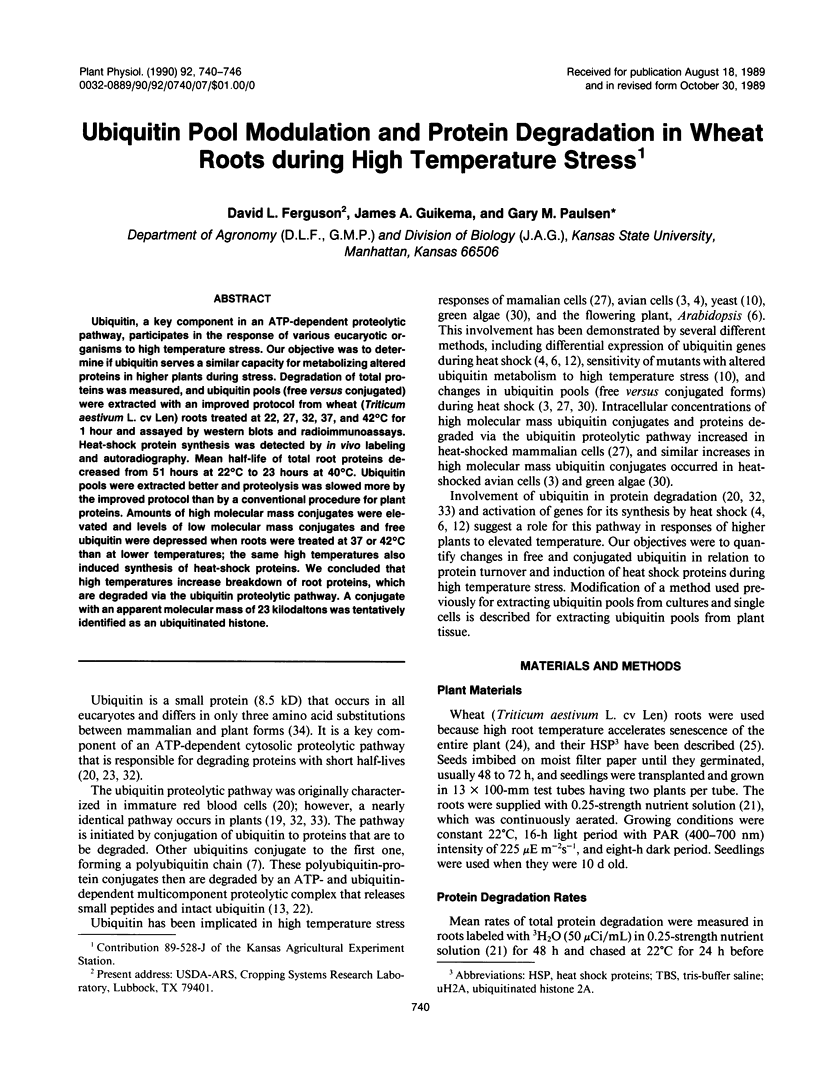



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Armond P. A., Björkman O., Staehelin L. A. Dissociation of supramolecular complexes in chloroplast membranes. A manifestation of heat damage to the photosynthetic apparatus. Biochim Biophys Acta. 1980 Oct 2;601(3):433–443. doi: 10.1016/0005-2736(80)90547-7. [DOI] [PubMed] [Google Scholar]
- Bond U., Agell N., Haas A. L., Redman K., Schlesinger M. J. Ubiquitin in stressed chicken embryo fibroblasts. J Biol Chem. 1988 Feb 15;263(5):2384–2388. [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. The chicken ubiquitin gene contains a heat shock promoter and expresses an unstable mRNA in heat-shocked cells. Mol Cell Biol. 1986 Dec;6(12):4602–4610. doi: 10.1128/mcb.6.12.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke T. J., Callis J., Vierstra R. D. Characterization of a polyubiquitin gene from Arabidopsis thaliana. Mol Gen Genet. 1988 Aug;213(2-3):435–443. doi: 10.1007/BF00339613. [DOI] [PubMed] [Google Scholar]
- Chau V., Tobias J. W., Bachmair A., Marriott D., Ecker D. J., Gonda D. K., Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989 Mar 24;243(4898):1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Greenwood J. S. Heat stress enhances phytohemagglutinin synthesis but inhibits its transport out of the endoplasmic reticulum. Plant Physiol. 1987 Apr;83(4):778–784. doi: 10.1104/pp.83.4.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D., Ozkaynak E., Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation, and other stresses. Cell. 1987 Mar 27;48(6):1035–1046. doi: 10.1016/0092-8674(87)90711-2. [DOI] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C., Lamoreaux E. Ubiquitin mRNA is a major stress-induced transcript in mammalian cells. Nucleic Acids Res. 1989 Feb 11;17(3):1215–1230. doi: 10.1093/nar/17.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganoth D., Leshinsky E., Eytan E., Hershko A. A multicomponent system that degrades proteins conjugated to ubiquitin. Resolution of factors and evidence for ATP-dependent complex formation. J Biol Chem. 1988 Sep 5;263(25):12412–12419. [PubMed] [Google Scholar]
- Goldknopf I. L., Busch H. N-Bromosuccinimide fragments of protein A24 (uH2A): an implication that ubiquitin is the precursor of conjugation in vivo. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1724–1731. doi: 10.1016/0006-291x(80)91373-x. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., Taylor C. W., Baum R. M., Yeoman L. C., Olson M. O., Prestayko A. W., Busch H. Isolation and characterization of protein A24, a "histone-like" non-histone chromosomal protein. J Biol Chem. 1975 Sep 25;250(18):7182–7187. [PubMed] [Google Scholar]
- Haas A. L., Bright P. M. The immunochemical detection and quantitation of intracellular ubiquitin-protein conjugates. J Biol Chem. 1985 Oct 15;260(23):12464–12473. [PubMed] [Google Scholar]
- Haas A. L., Murphy K. E., Bright P. M. The inactivation of ubiquitin accounts for the inability to demonstrate ATP, ubiquitin-dependent proteolysis in liver extracts. J Biol Chem. 1985 Apr 25;260(8):4694–4703. [PubMed] [Google Scholar]
- Hershko A. Ubiquitin-mediated protein degradation. J Biol Chem. 1988 Oct 25;263(30):15237–15240. [PubMed] [Google Scholar]
- Hough R., Pratt G., Rechsteiner M. Ubiquitin-lysozyme conjugates. Identification and characterization of an ATP-dependent protease from rabbit reticulocyte lysates. J Biol Chem. 1986 Feb 15;261(5):2400–2408. [PubMed] [Google Scholar]
- Jabben M., Shanklin J., Vierstra R. D. Ubiquitin-phytochrome conjugates. Pool dynamics during in vivo phytochrome degradation. J Biol Chem. 1989 Mar 25;264(9):4998–5005. [PubMed] [Google Scholar]
- Necchi A., Pogna N. E., Mapelli S. Early and late heat shock proteins in wheats and other cereal species. Plant Physiol. 1987 Aug;84(4):1378–1384. doi: 10.1104/pp.84.4.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel B. E., Allis C. D., Davie J. R. Ubiquitinated histone H2B is preferentially located in transcriptionally active chromatin. Biochemistry. 1989 Feb 7;28(3):958–963. doi: 10.1021/bi00429a006. [DOI] [PubMed] [Google Scholar]
- Parag H. A., Raboy B., Kulka R. G. Effect of heat shock on protein degradation in mammalian cells: involvement of the ubiquitin system. EMBO J. 1987 Jan;6(1):55–61. doi: 10.1002/j.1460-2075.1987.tb04718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijven A. H. Heat inactivation of starch synthase in wheat endosperm tissue. Plant Physiol. 1986 Jun;81(2):448–453. doi: 10.1104/pp.81.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. A., Bain J. L., Ellis S., Haas A. L. Quantitation and immunocytochemical localization of ubiquitin conjugates within rat red and white skeletal muscles. J Histochem Cytochem. 1988 Jun;36(6):621–632. doi: 10.1177/36.6.2835410. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Vierstra R. D. Demonstration of ATP-Dependent, Ubiquitin-Conjugating Activities in Higher Plants. Plant Physiol. 1987 Jun;84(2):332–336. doi: 10.1104/pp.84.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]