Abstract
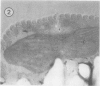
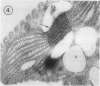
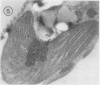
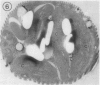
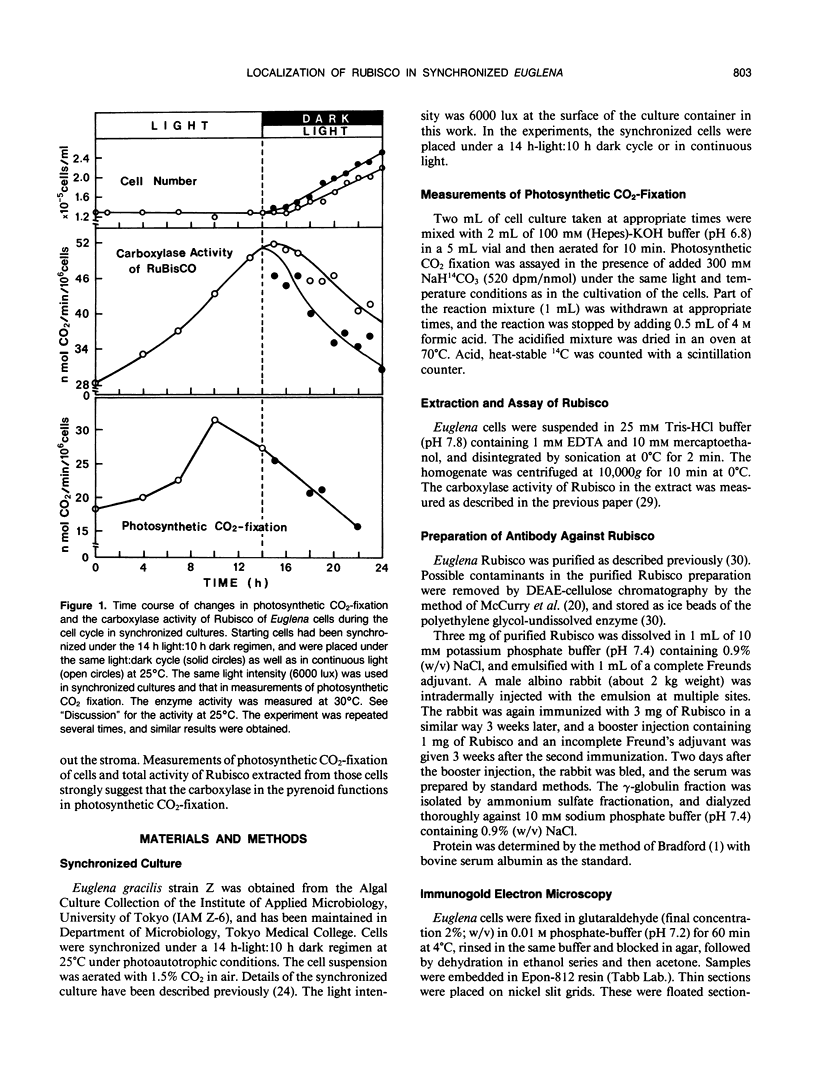
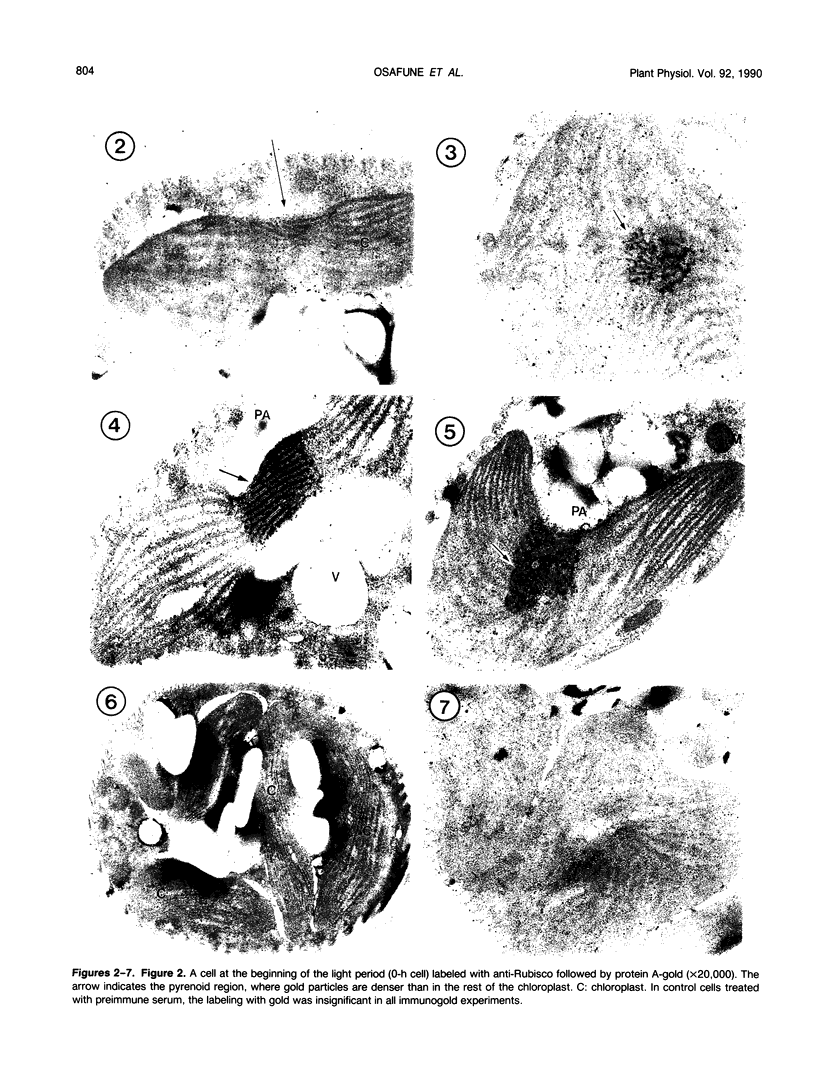
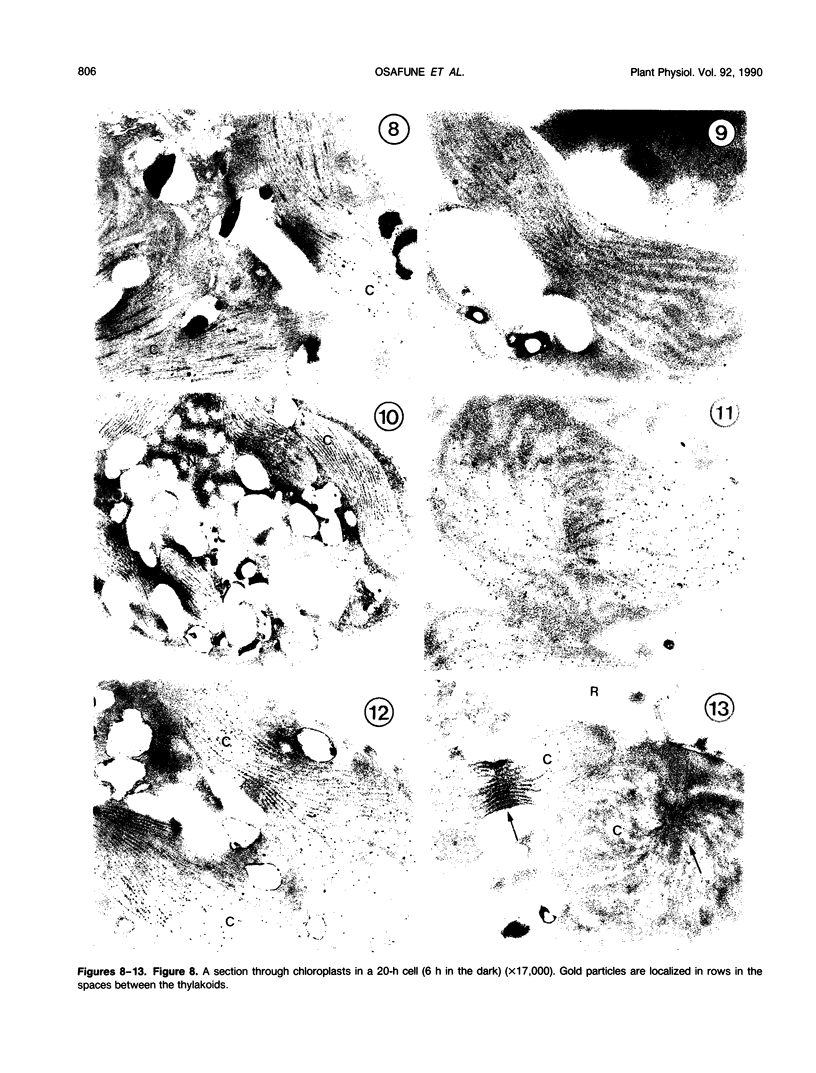
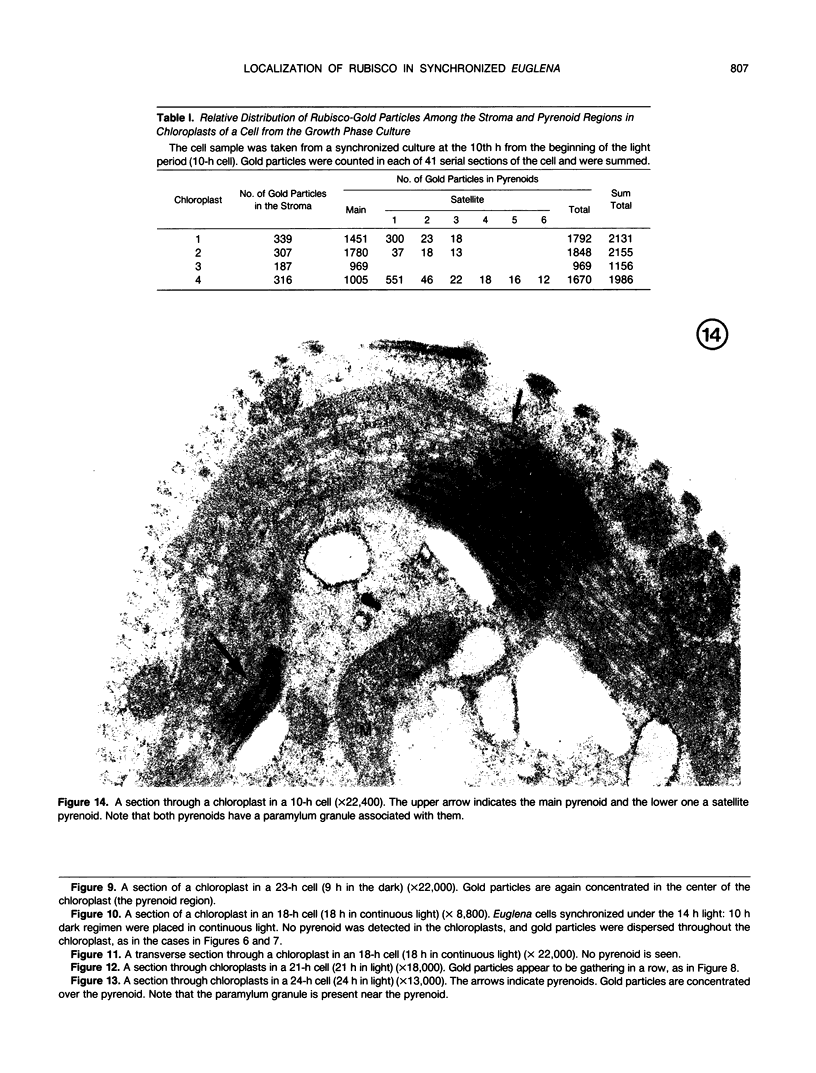
Euglena gracilis strain (Z) cells were synchronized under photoautotrophic conditions using a 14 hour light:10 hour dark regimen. The cells grew during the light period (growth phase) and divided during the following 10 hour period either in the dark or in the light (division phase). Changes in morphology of the pyrenoid and in the distribution of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) within the chloroplasts were followed by immunoelectron microscopy during the growth and division phases of Euglena cells. Epon-embedded sections were labeled with an antibody to the holoenzyme followed by protein A-gold. The immunoreactive proteins were concentrated in the pyrenoid, and less densely distributed in the stroma during the growth phase. During the division phase, the pyrenoid could not be detected and the gold particles were dispersed throughout the stroma. Toward the end of the division phase, the pyrenoid began to form in the center of a chloroplast, and the immunoreactive proteins started to concentrate over that rudimentary pyrenoid. During the growth phase, small areas rich in gold particles, called `satellite pyrenoid,' were observed, in addition to the main pyrenoid. From a comparison of photosynthetic CO2-fixation with the total carboxylase activity of Rubisco extracted from Euglena cells in the growth phase, it is suggested that the carboxylase in the pyrenoid functions in CO2-fixation in photosynthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Challis J. R., Harrison F. A., Heap R. B. The kinetics of oestradiol -17 metabolism in the sheep. J Endocrinol. 1973 Apr;57(1):97–110. doi: 10.1677/joe.0.0570097. [DOI] [PubMed] [Google Scholar]
- Cook J. R., Haggard S. S., Harris P. Cyclic changes in chloroplast structure in synchronized Euglena gracilis. J Protozool. 1976 Aug;23(3):368–373. doi: 10.1111/j.1550-7408.1976.tb03790.x. [DOI] [PubMed] [Google Scholar]
- Holdsworth R. H. The isolation and partial characterization of the pyrenoid protein of Eremosphaera viridis. J Cell Biol. 1971 Nov;51(21):499–513. doi: 10.1083/jcb.51.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste-Royal G., Gibbs S. P. Immunocytochemical Localization of Ribulose-1,5-Bisphosphate Carboxylase in the Pyrenoid and Thylakoid Region of the Chloroplast of Chlamydomonas reinhardtii. Plant Physiol. 1987 Mar;83(3):602–606. doi: 10.1104/pp.83.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ruiz A., Verbelen J. P., Roldan J. M., Diez J. Nitrate reductase of green algae is located in the pyrenoid. Plant Physiol. 1985 Dec;79(4):1006–1010. doi: 10.1104/pp.79.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry S. D., Gee R., Tolbert N. E. Ribulose-1,5-bisphosphate carboxylase/oxygenase from spinach, tomato, or tobacco leaves. Methods Enzymol. 1982;90(Pt E):515–521. doi: 10.1016/s0076-6879(82)90178-1. [DOI] [PubMed] [Google Scholar]
- Yokota A., Harada A., Kitaoka S. Characterization of ribulose 1,5-bisphosphate carboxylase/oxygenase from Euglena gracilis Z. J Biochem. 1989 Mar;105(3):400–405. doi: 10.1093/oxfordjournals.jbchem.a122676. [DOI] [PubMed] [Google Scholar]