Abstract
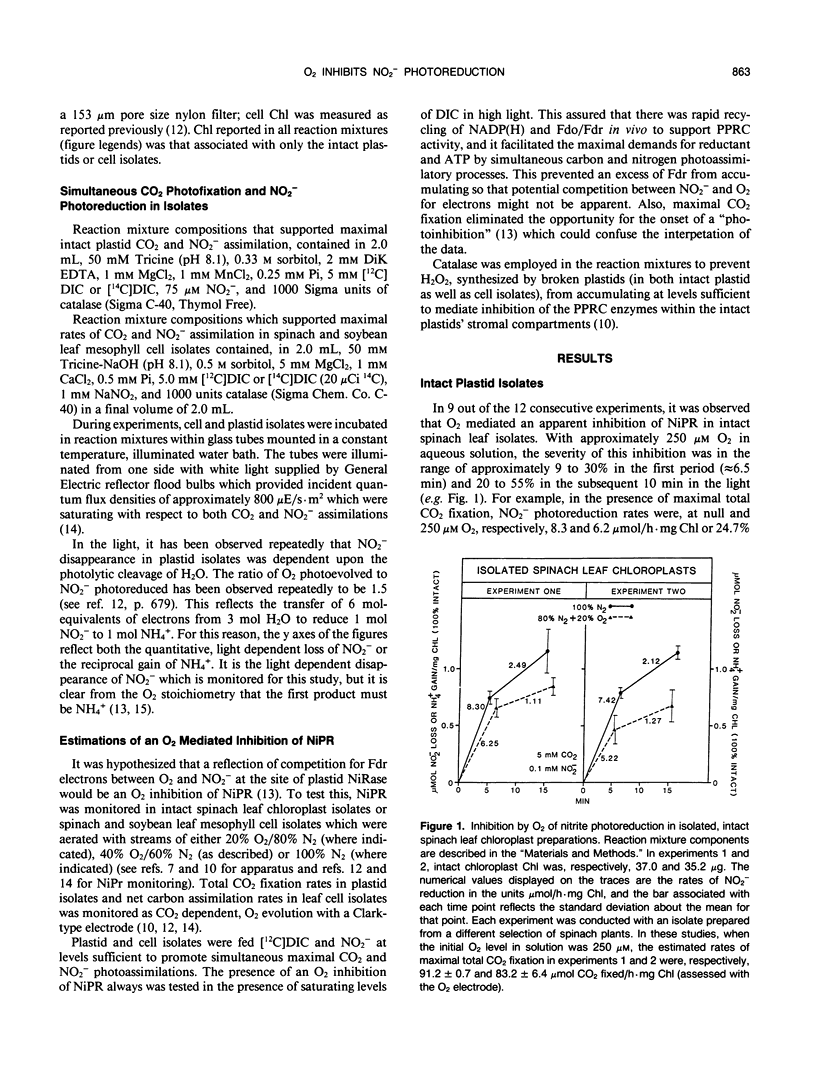
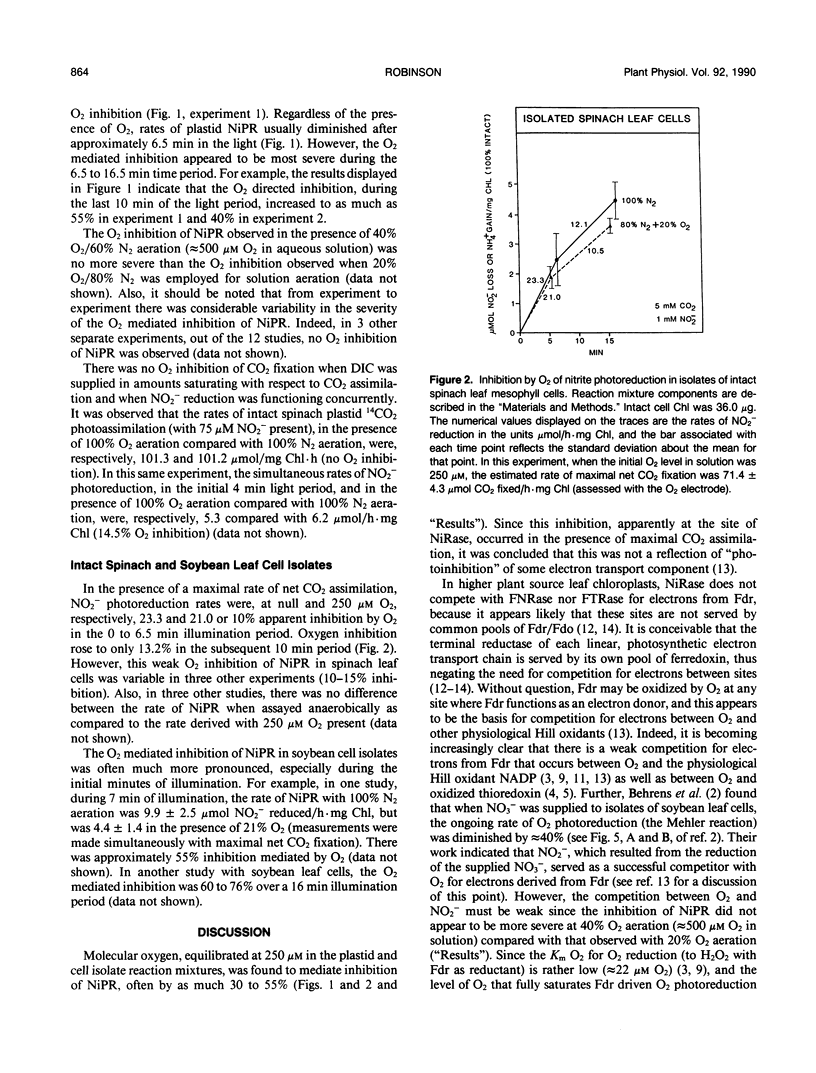
It was hypothesized previously that an O2 inhibition of NO2− photoreduction would reflect a competition between O2 and NO2− for electrons from ferredoxin at the site of plastid nitrite reductase. In order to test this in vivo, intact spinach (Spinacia oleracea L.) leaf chloroplast and mesophyll cell isolates held in high light were aerated with streams of 20% O2/80% N2 (250 micromolar O2 in aqueous solution) or, alternatively, streams of 100% N2. Bicarbonate plus CO2 and NO2− were supplied to reaction mixtures at levels just sufficient to promote maximal assimilations of CO2 and NO2−. In chloroplast isolates, there was a 9 to 30% O2 inhibition of NO2− reduction while there were high rates of CO2 fixation. In spinach and soybean (Glycine max) leaf cell isolates, NO2− photoreduction rates were 10 to 55% inhibited by O2 at near ambient levels. It is possible that O2 may compete, albeit weakly, with NO2− (nitrite reductase) for equivalents derived from reduced ferredoxin. Also, O2 may oxidize sulfhydryl groups on nitrite reductase which are involved in substrate binding and/or activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens P. W., Marsho T. V., Radmer R. J. Photosynthetic o(2) exchange kinetics in isolated soybean cells. Plant Physiol. 1982 Jul;70(1):179–185. doi: 10.1104/pp.70.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquot J. P., Gadal P., Nishizawa A. N., Yee B. C., Crawford N. A., Buchanan B. B. Enzyme regulation in C4 photosynthesis: mechanism of activation of NADP-malate dehydrogenase by reduced thioredoxin. Arch Biochem Biophys. 1984 Jan;228(1):170–178. doi: 10.1016/0003-9861(84)90058-4. [DOI] [PubMed] [Google Scholar]
- Robinson J. M. Carbon dioxide and nitrite photoassimilatory processes do not intercompete for reducing equivalents in spinach and soybean leaf chloroplasts. Plant Physiol. 1986 Mar;80(3):676–684. doi: 10.1104/pp.80.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M. Hydrogen peroxide synthesis in isolated spinach chloroplast lamellae : an analysis of the mehler reaction in the presence of NADP reduction and ATP formation. Plant Physiol. 1982 Nov;70(5):1249–1254. doi: 10.1104/pp.70.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M. Photosynthetic intermediates, the warburg effect, and glycolate synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Jun;53(6):790–797. doi: 10.1104/pp.53.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Smith M. G., Gibbs M. Influence of Hydrogen Peroxide upon Carbon Dioxide Photoassimilation in the Spinach Chloroplast: I. HYDROGEN PEROXIDE GENERATED BY BROKEN CHLOROPLASTS IN AN "INTACT" CHLOROPLAST PREPARATION IS A CAUSAL AGENT OF THE WARBURG EFFECT. Plant Physiol. 1980 Apr;65(4):755–759. doi: 10.1104/pp.65.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M. Spinach Leaf Chloroplast CO(2) and NO(2) Photoassimilations Do Not Compete for Photogenerated Reductant: Manipulation of Reductant Levels by Quantum Flux Density Titrations. Plant Physiol. 1988 Dec;88(4):1373–1380. doi: 10.1104/pp.88.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


