Abstract
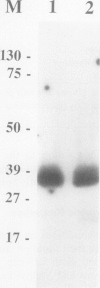
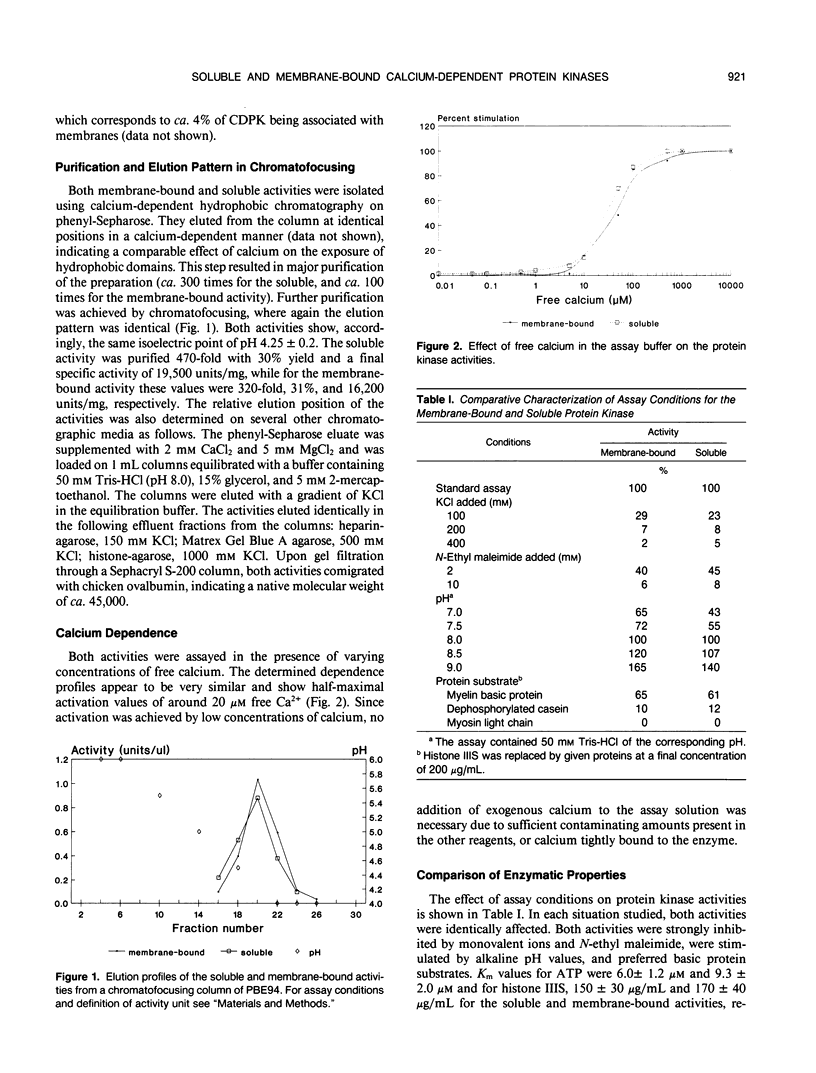
The soluble and membrane-bound forms of the calcium-dependent protein kinase from barley leaves (Hordeum vulgare L. cv. Borsoy) have been partially purified and compared. Both forms showed an active polypeptide of 37 kilodaltons on activity gels with incorporated histone as substrate. They eluted from chromatofocusing columns at an identical isoelectric point of pH 4.25 ± 0.2, and also comigrated on several other chromatographic affinity media including Matrex Gel Blue A, histone-agarose, phenyl-Sepharose, and heparin-agarose. Both activities comigrated with chicken ovalbumin during gel filtration through Sephacryl S-200, indicating a native molecular mass of 45 kilodaltons. The activities share a number of enzymatic properties including salt and pH dependence, free calcium stimulation profile, substrate specificity, and Km values. The soluble activity was shown to bind to artificial lipid vesicles. These data suggest strongly that the soluble and membrane-bound calcium-dependent protein kinases from barley are very closely related or even identical.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blowers D. P., Trewavas A. J. Autophosphorylation of plasma membrane bound calcium calmodulin dependent protein kinase from pea seedlings and modification of catalytic activity by autophosphorylation. Biochem Biophys Res Commun. 1987 Mar 13;143(2):691–696. doi: 10.1016/0006-291x(87)91409-4. [DOI] [PubMed] [Google Scholar]
- Blowers D. P., Trewavas A. J. Rapid cycling of autophosphorylation of a ca-calmodulin regulated plasma membrane located protein kinase from pea. Plant Physiol. 1989 Aug;90(4):1279–1285. doi: 10.1104/pp.90.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geahlen R. L., Anostario M., Jr, Low P. S., Harrison M. L. Detection of protein kinase activity in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1986 Feb 15;153(1):151–158. doi: 10.1016/0003-2697(86)90074-6. [DOI] [PubMed] [Google Scholar]
- Harmon A. C., Putnam-Evans C., Cormier M. J. A calcium-dependent but calmodulin-independent protein kinase from soybean. Plant Physiol. 1987 Apr;83(4):830–837. doi: 10.1104/pp.83.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucis E., Polya G. M. Calcium-independent activation of two plant leaf calcium-regulated protein kinases by unsaturated fatty acids. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1041–1047. doi: 10.1016/s0006-291x(87)80175-4. [DOI] [PubMed] [Google Scholar]
- Klucis E., Polya G. M. Localization, solubilization and characterization of plant membrane-associated calcium-dependent protein kinases. Plant Physiol. 1988 Sep;88(1):164–171. doi: 10.1104/pp.88.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McDonnel A., Staehelin L. A. Adhesion between liposomes mediated by the chlorophyll a/b light-harvesting complex isolated from chloroplast membranes. J Cell Biol. 1980 Jan;84(1):40–56. doi: 10.1083/jcb.84.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjeva R., Refeno G., Boudet A. M., Marmé D. Activation of plant quinate:NAD 3-oxidoreductase by Ca and calmodulin. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5222–5224. doi: 10.1073/pnas.80.17.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]