Abstract
The worldwide increase in the incidence of metabolic syndrome correlates with marked increase in total fructose intake in the form of high-fructose corn syrup, beverage and table sugar. Increased dietary fructose intake in rodents has been shown to recapitulate many aspects of metabolic syndrome by causing hypertension, insulin resistance, and hyperlipidemia. Recent studies demonstrated that increased dietary fructose intake stimulates salt absorption in the small intestine and kidney tubules, resulting in a state of salt overload and thus causing hypertension. The absorption of salt (sodium and chloride) in the small intestine is predominantly mediated via the chloride/base exchangers DRA (SLC26A3) and PAT1 (SLC26A6), and the Na+/H+ exchanger NHE3 (SLC9A3). PAT1 and NHE3 also co-localize on the apical membrane of kidney proximal tubule. Luminal fructose stimulated salt absorption in the jejunum and kidney tubules, responses that were significantly diminished in PAT1 null mice. These studies further demonstrated that Glut5 (SLC2A5) is the major fructose-absorbing transporter in the small intestine (and kidney proximal tubule) and plays an essential role in the systemic homeostasis of fructose. Increased dietary fructose intake for several weeks upregulated the expression of NHE3, PAT1 and Glut5 in the intestine and resulted in hypertension in wild type mice, a response that was almost abolished in PAT1 null mice and abrogated in Glut5 null mice. This article will discuss the interaction of Glut5 with salt absorbing transporters and review the role of dietary fructose in enhanced salt absorption in intestine and kidney as it relates to the pathogenesis of hypertension in metabolic syndrome.
Keywords: Glut5, DRA, PAT1, NHE3, fructose-stimulated salt absorption, Glut2
Overview and Discussion
Metabolic Syndrome and Hypertension
Metabolic syndrome is manifested by visceral obesity, glucose intolerance, hypertension, hyperinsulinism and atherogenic dyslipidemia (1–6). The prevalence of obesity and metabolic syndrome is reaching epidemic proportions in adults and school age adolescents and children, specifically in United States, and portends ominously for the future health of people in both developed and developing countries (7). There is no universal agreement as to the underlying pathophysiology of metabolic syndrome.
At its core, the metabolic syndrome is the result of energy excess; therefore treating obesity is a good strategy to reverse the clinical features of the metabolic syndrome. While the increase in the incidence of obesity has been attributed to increased calorie intake and decreased physical activity, the pathogenesis of hypertension in metabolic syndrome remains less well understood. Possibilities such as activation of renin angiotensin system, enhanced insulin secretion, obesity, elevated serum uric acid and decreased nitric oxide generation by vascular endothelial cells have been implicated, and evidence in support of their role in the generation of hypertension has been presented (8–10). For example, insulin resistance and compensatory hyperinsulinemia have been shown to play a role in blood pressure elevation. Separate studies have implicated sodium retention, sympathetic activation and impairment of endothelial nitric oxide production as pathogenic factors in the generation of hypertension (11, 12). In addition, increased expression of angiotensin II type 1 receptor in mesangial cells has been suggested to play an important role in the pathogenesis of hypertension in metabolic syndrome (13). Recent studies have also implicated stress response, with an underlying abnormality in the enzyme 11beta-hydroxysteroid dehydrogenase (HSD1), as a contributing factor (14). At the cellular level, HSD1 locally regenerates active cortisol from inactive cortisone, amplifying glucocorticoid receptor activation and promoting preadipocyte differentiation and adipocyte hypertrophy (14, 15).
Metabolic Syndrome and increased dietary fructose intake
Americans are consuming around 22 teaspoons of sugar each day, the American Heart Association reports (7). A national health survey has shown that teens between ages 14 to 18 consume an astonishing 34 teaspoons of added sugar a day (7). Most of the added sugar comes from soft drinks and candy - a staggering 355 calories, and is predominantly in the form of fructose. The steep increase in fructose consumption directly correlates with the increased incidence of Metabolic Syndrome and prevalence of hypertension in developed countries (7).
In rats, mice and dogs, increased dietary fructose intake for several weeks has been shown to recapitulate many parameters of metabolic syndrome including hypertension, insulin resistance, and hyperlipidemia (16–25). Indeed, increased dietary fructose intake has been shown to cause hypertension in rats, as early as 2 to 4 weeks after the start of the experiment (16–22). While dogs develop hypertension as early as 2 weeks after the start of high fructose diet, it takes more than 10 weeks for mice to develop hypertension when fed increased dietary fructose (23–25).
Increased dietary fructose, high salt diet and hypertension
In addition to fructose, Americans consume two to three times the recommended amount of salt, according to the Center for Disease Control (CDC), which warns that increased salt intake is significantly raising the risk of hypertension, cardiovascular disease, and kidney failure (2009).
Hypertension is a complex, multi-factorial disorder and attributing its etiology to a single factor is probably simplistic. One major factor, however, which is essential to the understanding of blood pressure regulation and hypertension, is altered salt absorption in the kidney (26–29). Several studies have implicated enhanced salt intake and absorption in the kidney, in the context of high insulin levels, as important factors in the pathophysiology of hypertension in metabolic syndrome (30–32).
Given the role of increased dietary fructose or salt intake in causing hypertension independent of each other, and given the increased consumption of both fructose and salt in our daily diet, the question should be asked as to whether increased dietary fructose and salt have any additive effect on blood pressure elevation. The answer to this important question remains speculative. Recent studies have shed new light on the role of dietary fructose on salt absorption in the intestine or kidney, which will be discussed below.
Fructose absorption in the small intestine and kidney proximal tubule
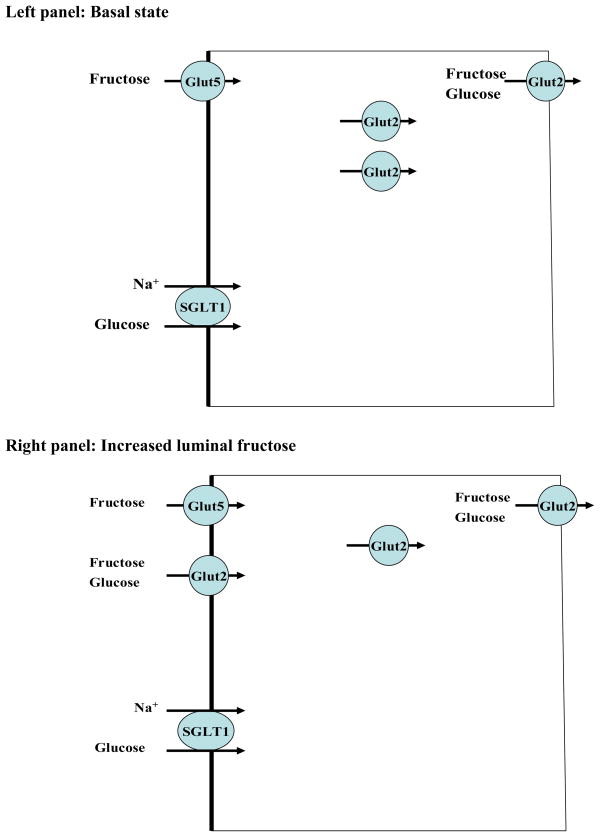
The absorption of fructose occurs in the small intestine and kidney proximal tubule, predominantly via members of facilitative carbohydrate transporters (Gluts). Both Glut2 and Glut5 are expressed in the small intestine and are able to transport fructose (33–39). The Schematic diagram 1 (left and right) depicts the localization and role of Glut2 and Glut5 in fructose absorption in jejunum at basal state and in response to increased dietary fructose intake. As indicated, Glut5 is expressed on the apical membrane of enterocytes whereas Glut2 is located intracellularly and on the basolateral membrane at basal state (left) and is recruited to the apical membrane in the presence of luminal fructose or glucose (right). Glut5 is detected in the apical membrane at both the basal state and in response to increased dietary fructose intake (Schematic diagram 1). It has been proposed that Glut2 is a major glucose and fructose-absorbing transporter, at least in the presence of increased dietary fructose or glucose intake. In addition to the small intestine, Glut5 is abundantly expressed in the proximal tubule, specifically in the S3 segment (40). Few studies have speculated that the absorption of fructose in the small intestine and kidney proximal tubule is mediated in part via Glut5 (Slc2a5).
Schematic diagram 1. Localization and role of Glut2 and Glut5 in fructose absorption in jejunum at basal state (left panel) and in response to increased dietary fructose intake (right panel).
SGLT1 (sodium glucose cotransporter 1) facilitates glucose and sodium absorption. Glut2 can transport both glucose and fructose and is recruited to the apical membrane in the presence of increased dietary fructose or glucose intake. Glut5 only transports fructose and resides in the apical membrane at basal state and in response to increased dietary fructose intake.
Salt absorption in the small intestine and kidney proximal tubule
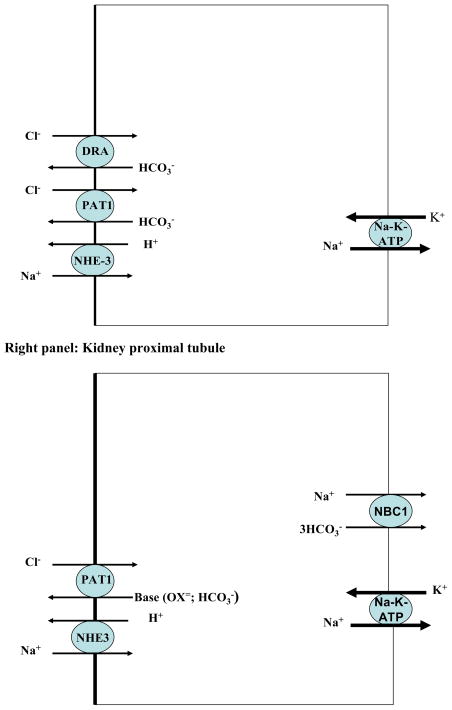
The absorption of salt in the small intestine is mediated via two apical Cl−/HCO3− exchangers DRA (Slc26a3) and PAT1 (Slc26a6) working in coordination with the Na+/H+ exchanger NHE3 (Slc9a3) (41–56). The Schematic diagram 2 (left and right) depicts the localization of salt absorbing transporters in the small intestine (left) and kidney proximal tubule (right). As shown, SLC26A6 (human)/Slc26a6 (mouse), also known as PAT1 (Putative Anion Transporter 1) or CFEX (chloride/formate exchanger), is expressed on the apical membrane of upper villous epithelium in small intestine and kidney proximal tubule (44–52). PAT1, which can function in Cl−/HCO3− and Cl−/oxalate exchange modes in vivo, plays an important role in the absorption of salt and secretion of bicarbonate in the small intestine (44–52). PAT1 also plays an important role in chloride absorption in the kidney proximal tubule (45).
Schematic diagram 2. Localization of salt absorbing transporters in the small intestine (left panel) and kidney proximal tubule (right panel).
NHE3 = Sodium Hydrogen Exchanger3= SLC9A3; PAT1 = Putative Anion Transporter 1= SLC26A6; DRA = Down Regulated in Adenoma =SLC26A3; NBC1: Sodium:bicarbonate cotransporter1 =SLC4A4
DRA (Slc26a3) is expressed on the apical membrane of mid to lower villous epithelium in small intestine and upper villous epithelium of large intestine (53–55). DRA plays an essential role in chloride absorption in the small and large intestines and its mutation inactivation in human results in chloride-losing diarrhea (53). Genetic deletion of DRA recapitulates the phenotype of chloride losing diarrhea in mice (54).
The sodium absorption in the small intestine as well as the kidney proximal tubule is mediated predominantly via NHE3 (left and right panels) (41, 56, 57). In addition to NHE3, the Na+/H+ exchanger NHE2 is also expressed on the apical membrane domain of enterocytes in the small intestine, but it does not seem to play a major role in sodium absorption in mammalians. As shown in the right panel (Schematic diagram 2), PAT1 and NHE3 also co-localize on the apical membrane of kidney proximal tubule (41, 51, 52, 56, 57).
Glut5 and PAT1 in the small intestine: interaction and localization
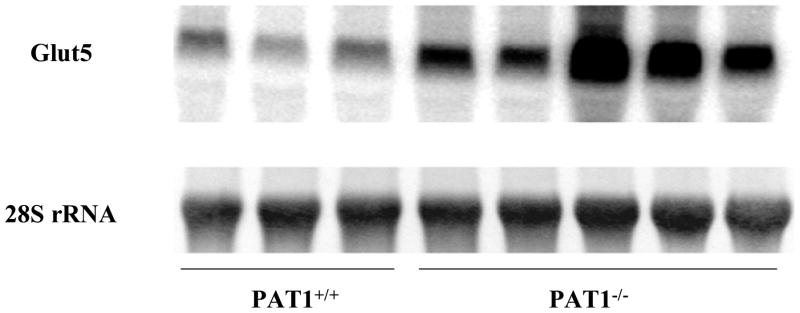
DNA microarray experiments revealed that a total of 27 genes were upregulated by at least twofold in the small intestine of PAT1−/− mice (personal observation). The most notable was that of Glut5 (Slc2a5), a fructose transporter which showed a ~fivefold upregulation (Fig. 1). As shown, mRNA expression of Glut5 increased significantly in jejunum of PAT1 ko mice (Figure 1). Glut5 shows similar pattern of expression in jejuna of PAT1 wt and ko mice (25). Immunofluorescence labeling indicated apical co-localization of Glut5 and PAT1 on jejunal villi in wt mice (25).
Figure 1. Expression of Glut5 increased significantly in jejunum of PAT1 ko mice.
Northern hybridizations verified the results of DNA microarray and demonstrated that the expression of Glut5 increased by ~5 folds in jejunum of PAT1 null mice.
Fructose stimulates salt absorption in the jejunum predominantly via PAT1 activation
Given the co-localization of Glut5 and PAT-1 (25) and the upregulation of Glut5 mRNA in the small intestine of PAT1−/− mice (Fig. 1), we entertained the possibility that fructose may stimulate salt absorption by activating PAT1. To test this possibility, proximal jejunum was perfused in vivo with isotonic perfusate and net fluid absorption was examined, before and after the addition of fructose (25). The results demonstrated that fructose at 40 mM elicited a significant increase in fluid absorption in PAT1+/+ mice (p<0.01, n=5), a response which was blunted in PAT1−/− mice (25). The removal of chloride from the perfusate inhibited the basal fluid absorption by 75%, and completely abrogated the fructose-stimulated fluid absorption in PAT1+/+ mice (25).
Contrary to the blunted response in jejunum of PAT1 ko mice, luminal fructose elicited a very robust stimulatory effect on salt absorption in jejunum of DRA ko mice (Seidler, Soleimani communication), indicating that DRA is not the target of fructose action in jejunum. The fructose-stimulated salt absorption in jejunum was completely abrogated in NHE3 ko mice (Seidler, Soleimani communication). Taken together, these studies strongly suggest that fructose-stimulated salt absorption is mediated via PAT1 and NHE3 working in parallel.
Increased fructose intake enhances the expression of Glut5, PAT1, and NHE3 in the small intestine
The above studies in perfused jejunum were acute and the onset of the effect of fructose was observed in a matter of minutes. To examine the role of long term consumption of fructose on ion transporters, animals were fed 60% fructose diet and compared to 60% starch as control. Increased dietary fructose intake (60% fructose) for two weeks enhanced the mRNA expression and protein abundance of Glut5, PAT1, and NHE3 in jejunum Vs. control diet (60% starch) (25). The two-week time point was chosen because animals had not yet developed hypertension (personal observation).
Increased dietary fructose intake decreases urinary salt excretion in rats and mice
To examine the role of dietary fructose on renal salt excretion balanced studies were performed in rats and mice before and after switching to high fructose diet. The results demonstrated that high fructose diet significantly decreased the daily excretion of chloride and sodium in rat kidney (25). The kidney function, including BUN and serum creatinine and urine osmolarity remained unchanged (25). The stimulatory effect of high fructose diet on salt absorption in the kidney was also evident in wild-type (PAT1+/+) mice. However, PAT1 ko mice displayed enhanced kidney salt excretion when subjected to increased dietary fructose intake. The sodium and chloride excretion rates in PAT1 wt and null mice on control diet were comparable and significantly lower than PAT1 null mice on high fructose diet. Kidney function and serum uric acid remained normal and comparable in PAT+/+ and PAT−/− mice on high fructose diet, indicating the absence of dehydration or any correlation between increased dietary fructose intake and serum uric acid levels.
Fructose-induced hypertension: Role of salt absorbing transporters in intestine and kidney
To determine the impact of fructose-stimulated salt absorption on blood pressure, systolic blood pressure was measured in conscious mice by tail cuff method. The results indicated that PAT1+/+ mice on a high fructose diet for 12 weeks developed significant increase in their blood pressure Vs. control diet (25). The systolic blood pressure in PAT1+/+ mice increased from 102 ± 2 mm Hg on a normal diet to 111 ± 2.2 on a high fructose diet (p<0.02) (25). However, PAT1−/− mice failed to develop hypertension on a high fructose diet (25). PAT1−/− mice displayed normal food intake and weight gain on either diet Vs. PAT1+/+ mice. Blood sugar increased in both PAT1+/+ and PAT1−/− mice on increased dietary fructose intake (25). The increase in plasma fructose, measured by HPLC, was comparable in both PAT1+/+ and PAT1−/− mice on increased dietary fructose (25).
Fructose absorption in the small intestine and fructose-induced hypertension are absolutely dependent on Glut5
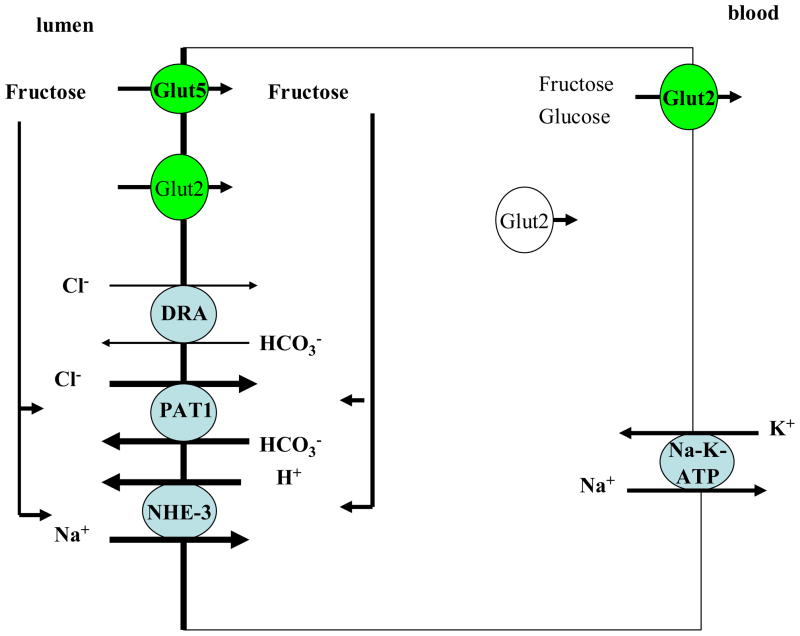
Glut5 transports fructose; whereas, Glut2 transports both glucose and fructose (schematic diagram 1). To ascertain the role of Glut5 in fructose absorption, animals with genetic deletion of Glut5 were placed on high fructose diet and compared to control diet (58). Our results indicated that increased dietary fructose intake (60% fructose) in Glut5 ko mice causes severe small intestinal malabsorption, as evident by massive dilation of bowel loops and specifically caecum as early as 72 hours after the start of fructose-containing diet (58). Glut5+/+ mice showed a six-fold increase in their blood fructose levels (58). However, Glut5−/− mice did not show any noticeable increase in their blood fructose level after five days of high fructose diet (58). Glut5−/− mice develop hypotension after 5 days of increased dietary fructose intake, due to volume depletion (58). Interestingly, Glut5−/− mice did not demonstrate any fructose-stimulated salt absorption in their jejuna (58), indicating that presence of Glut5 is essential for the fructose-stimulated salt absorption in the intestine. Schematic diagram 3 depicts the role of fructose and Glut5 in fructose absorption and fructose-stimulated salt absorption in the small intestine..
Schematic diagram 3. The role of fructose and Glut5 in fructose absorption and fructose-stimulated salt absorption in the small intestine.
Luminal fructose, via Glut5 action, stimulates PAT1 and NHE3, therefore enhancing salt absorption in the jejunum. The stimulatory effect of luminal fructose on salt absorption in jejunum was completely abrogated in Glut5 null mice (ref. 58).
The absence of hyperuricema in early stages of fructose-induced hypertension
Our studies in rats on high fructose diet for five weeks or in mice on high fructose diet for 12 weeks did not demonstrate any elevation in serum uric acid; however, they showed enhanced excretion of uric acid in the urine (25). These results clearly demonstrate that serum uric acid is not causally linked to the generation of hypertension in fructose-induced hypertension.
Role of increased dietary salt intake and gender in fructose-induced hypertension
Recent studies in our laboratories have indicated that increased dietary fructose intake accelerated the onset of hypertension relative to normal salt intake, an effect that was more pronounced in male mice (personal observation). Our results further demonstrate that increased dietary fructose intake decreased the concentration of urinary cGMP in animals on either normal or high salt diet, suggesting the impairment of the natriuretic crosstalk between intestine and kidney.
It is widely believed that enhanced salt absorption in the intestine per se should not in and out of itself lead to a state of salt overload, if the kidney retains its ability to excrete the excess salt. However, it should be noted that the same salt absorbing transporters in the small intestine (namely PAT1 and NHE3) are also expressed in the proximal tubule (Schematic diagram 2). As such, increased dietary fructose intake will likely enhance salt absorption in both organs by activating PAT1 (and NHE3), therefore preventing the kidney from excreting the excess salt load. In support of the role of salt excess in the pathogenesis of fructose-mediated hypertension, studies in rats demonstrated that low salt diet completely abrogated fructose-stimulated hypertension (59).
Summary and Conclusions
Increased dietary fructose intake stimulates salt and fructose absorption in the small intestine and kidney proximal tubule through coordinated activation of PAT1, NHE3, and Glut5. PAT1, NHE3 or Glut5 deletion blunts fructose-stimulated salt absorption in the small intestine. Deletion of PAT1 also blocks the stimulatory effect of fructose on salt absorption in the kidney proximal tubule. Further, fructose-induced hypertension was almost abolished in PAT1 ko mice on high fructose diet.
The exact mechanism(s) linking fructose and Glut5 to PAT1 (and NHE3) stimulation in the small intestine remains speculative. The absence of any stimulatory effect of fructose on salt absorption in Glut5 KO mice intestine strongly suggests that fructose exerts its stimulatory effect after it is being absorbed via Glut5. Whether, the absorbed fructose changes the electrochemical gradients for salt absorption across the luminal membrane or directly activates PAT1 (and NHE3) through signaling pathways remains speculative.
Taken together, these results strongly suggest that fructose-induced hypertension is generated in large part by an state of salt overload resulting from enhanced salt absorption in intestine and kidney. We propose that reducing dietary intake of fructose and salt as well as maneuvers aimed at inhibiting salt absorption in the intestine and kidney tubules could have profound beneficial effect on controlling blood pressure in patients with metabolic syndrome.
Acknowledgments
These studies that were performed in the PI’s laboratory were supported by grants from the Department of Veterans Affairs and the National Institute of Health Grant (DK 62809), and by grants from DCI and DCA dialysis care group.
Footnotes
CONFLICTS OF INTEREST
There is no conflicts of interst.
References
- 1.Sacks FM. Metabolic syndrome: epidemiology and consequences. J Clin Psychiatry. 2004;65(Suppl 18):3–12. Review. [PubMed] [Google Scholar]
- 2.Spinler SA. Challenges associated with metabolic syndrome. Pharmacotherapy. 2006;26(12 Pt 2):209S–217S. doi: 10.1592/phco.26.12part2.209S. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92(2):399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- 4.Sinaiko A. Obesity, insulin resistance and the metabolic syndrome. J Pediatr (Rio J) 2007;83(1):3–4. doi: 10.2223/JPED.1585. [DOI] [PubMed] [Google Scholar]
- 5.Reusch JE. Current concepts in insulin resistance, type 2 diabetes mellitus, and the metabolic syndrome. Am J Cardiol. 2002;90(5A):19G–26G. doi: 10.1016/s0002-9149(02)02555-9. Review. [DOI] [PubMed] [Google Scholar]
- 6.Stuhlinger MC, Abbasi F, Chu JW, Lamendola C, McLaughlin TL, Cooke JP, Reaven GM, Tsao PS. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA. 2002;287(11):1420–6. doi: 10.1001/jama.287.11.1420. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism and the Council on Epidemiology and Prevention. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120(11):1011–20. doi: 10.1161/CIRCULATIONAHA.109.192627. [DOI] [PubMed] [Google Scholar]
- 8.Cirillo P, Sato W, Reungjui S, Heinig M, Gersch M, Sautin Y, Nakagawa T, Johnson RJ. Uric Acid, the metabolic syndrome, and renal disease. J Am Soc Nephrol. 2006;17(12 Suppl 3):S165–8. doi: 10.1681/ASN.2006080909. [DOI] [PubMed] [Google Scholar]
- 9.Kagota S, Yamaguchi Y, Tanaka N, Kubota Y, Kobayashi K, Nejime N, Nakamura K, Kunitomo M, Shinozuka K. Disturbances in nitric oxide/cyclic guanosine monophosphate system in SHR/NDmcr-cp rats, a model of metabolic syndrome. Life Sci. 2006;78(11):1187–96. doi: 10.1016/j.lfs.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Sarafidis PA, Bakris GL. Insulin and endothelin: an interplay contributing to hypertension development? J Clin Endocrinol Metab. 2007;92(2):379–85. doi: 10.1210/jc.2006-1819. [DOI] [PubMed] [Google Scholar]
- 11.Huggett RJ, Burns J, Mackintosh AF, Mary DA. Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension. 2004;44(6):847–52. doi: 10.1161/01.HYP.0000147893.08533.d8. [DOI] [PubMed] [Google Scholar]
- 12.Grassi G, Seravalle G, Quarti-Trevano F, Scopelliti F, Dell’oro R, Bolla G, Mancia G. Excessive sympathetic activation in heart failure with obesity and metabolic syndrome: Characteristics and mechanisms. Hypertension. 2007 Jan 8; doi: 10.1161/01.HYP.0000255983.32896.b9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Karagiannis A, Mikhailidis DP, Athyros VG, Kakafika AI, Tziomalos K, Liberopoulos EN, Florentin M, Elisaf M. Angiotensin II as a multifunctional hormone: a regulator of haemodynamics and metabolism with potential roles in the etiologies of hypertension and the metabolic syndrome. J Hypertens. 2006;24(1):37–8. doi: 10.1097/01.hjh.0000198044.69999.31. [DOI] [PubMed] [Google Scholar]
- 14.Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–70. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 15.Sukhija R, Kakar P, Mehta V, Mehta JL. Enhanced 11beta-hydroxysteroid dehydrogenase activity, the metabolic syndrome, and systemic hypertension. Am J Cardiol. 2006;98(4):544–8. doi: 10.1016/j.amjcard.2006.03.028. Review. [DOI] [PubMed] [Google Scholar]
- 16.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76(5):911–22. doi: 10.1093/ajcn/76.5.911. Review. [DOI] [PubMed] [Google Scholar]
- 17.Giacchetti G, Sechi LA, Griffin CA, Don BR, Mantero F, Schambelan M. The tissue renin-angiotensin system in rats with fructose-induced hypertension: overexpression of type 1 angiotensin II receptor in adipose tissue. J Hypertens. 2000;18(6):695–702. doi: 10.1097/00004872-200018060-00006. [DOI] [PubMed] [Google Scholar]
- 18.Lee DH, Lee JU, Kang DG, Paek YW, Chung DJ, Chung MY. Increased vascular endothelin-1 gene expression with unaltered nitric oxide synthase levels in fructose-induced hypertensive rats. Metabolism. 2001;50(1):74–8. doi: 10.1053/meta.2001.19527. [DOI] [PubMed] [Google Scholar]
- 19.Catena C, Giacchetti G, Novello M, Colussi G, Cavarape A, Sechi LA. Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension. Am J Hypertens. 2003;16(11 Pt 1):973–8. doi: 10.1016/s0895-7061(03)01002-1. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh PS, Tai YH, Loh CH, Shih KC, Cheng WT, Chu CH. Functional interaction of AT1 and AT2 receptors in fructose-induced insulin resistance and hypertension in rats. Metabolism. 2005;54(2):157–64. doi: 10.1016/j.metabol.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Farah V, Elased KM, Morris M. Genetic and dietary interactions: role of angiotensin AT1a receptors in response to a high-fructose diet. Am J Physiol Heart Circ Physiol. 2007;293(2):H1083–9. doi: 10.1152/ajpheart.00106.2006. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Lozada LG, Tapia E, Jimenez A, Bautista P, Cristobal M, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007;292(1):F423–9. doi: 10.1152/ajprenal.00124.2006. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FJ, Rizza RA, Romero JC. High-fructose feeding elicits insulin resistance, hyperinsulinism, and hypertension in normal mongrel dogs. Hypertension. 1994;23(4):456–63. doi: 10.1161/01.hyp.23.4.456. [DOI] [PubMed] [Google Scholar]
- 24.Farah V, Elased KM, Morris M. Genetic and dietary interactions: role of angiotensin AT1a receptors in response to a high-fructose diet. Am J Physiol Heart Circ Physiol. 2007;293(2):H1083–9. doi: 10.1152/ajpheart.00106.2006. [DOI] [PubMed] [Google Scholar]
- 25.Singh AK, Amlal H, Haas PJ, Dringenberg U, Fussell S, Barone SL, Engelhardt R, Zuo J, Seidler U, Soleimani M. Fructose-induced hypertension: essential role of chloride and fructose absorbing transporters PAT1 and Glut5. Kidney Int. 2008;74(4):438–47. doi: 10.1038/ki.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Shaughnessy KM, Karet FE. Salt handling and hypertension. J Clin Invest. 2004;113(8):1075–81. doi: 10.1172/JCI21560. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85(2):679–715. doi: 10.1152/physrev.00056.2003. Review. [DOI] [PubMed] [Google Scholar]
- 28.Karppanen H, Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis. 2006;49(2):59–75. doi: 10.1016/j.pcad.2006.07.001. Review. [DOI] [PubMed] [Google Scholar]
- 29.Haddy FJ. Role of dietary salt in hypertension. Life Sci. 2006;79:1585–92. doi: 10.1016/j.lfs.2006.05.017. Review. [DOI] [PubMed] [Google Scholar]
- 30.Hall JE, Brands MW, Hildebrandt DA, Mizelle HL. Obesity-associated hypertension. Hyperinsulinemia and renal mechanisms. Hypertension. 1992 Jan;19(1 Suppl):I45–55. doi: 10.1161/01.hyp.19.1_suppl.i45. Review. [DOI] [PubMed] [Google Scholar]
- 31.Bjorntorp P, Holm G, Rosmond R, Folkow B. Hypertension and the metabolic syndrome: closely related central origin? Blood Press. 2000;9(2–3):71–82. doi: 10.1080/08037050050151762. Review. [DOI] [PubMed] [Google Scholar]
- 32.Ogihara T, Asano T, Fujita T. Contribution of salt intake to insulin resistance associated with hypertension. Life Sci. 2003;73(5):509–23. doi: 10.1016/s0024-3205(03)00315-1. Review. [DOI] [PubMed] [Google Scholar]
- 33.Ferraris RP. Dietary and developmental regulation of intestinal sugar transport. Biochem J. 2001;360:265–76. doi: 10.1042/0264-6021:3600265. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leturque A, Brot-Laroche E, Le Gall M, Stolarczyk E, Tobin V. The role of GLUT2 in dietary sugar handling. J Physiol Biochem. 2005;61:529–37. doi: 10.1007/BF03168378. Review. [DOI] [PubMed] [Google Scholar]
- 35.Gouyon F, Caillaud L, Carriere V, Klein C, Dalet V, Citadelle D, Kellett GL, Thorens B, Leturque A, Brot-Laroche E. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice. J Physiol. 2003;552:823–32. doi: 10.1113/jphysiol.2003.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kayano T, Burant CF, Fukumoto H, Gould GW, Fan YS, Eddy RL, Byers MG, Shows TB, Seino S, Bell GI. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6) J Biol Chem. 1990;265(22):13276–82. [PubMed] [Google Scholar]
- 37.Shu R, David ES, Ferraris RP. Dietary fructose enhances intestinal fructose transport and GLUT5 expression in weaning rats. Am J Physiol. 1997;272(3 Pt 1):G446–53. doi: 10.1152/ajpgi.1997.272.3.G446. [DOI] [PubMed] [Google Scholar]
- 38.Corpe CP, Basaleh MM, Affleck J, Gould G, Jess TJ, Kellett GL. The regulation of GLUT5 and GLUT2 activity in the adaptation of intestinal brush-border fructose transport in diabetes. Pflugers Arch. 1996;432(2):192–201. doi: 10.1007/s004240050124. [DOI] [PubMed] [Google Scholar]
- 39.Helliwell PA, Richardson M, Affleck J, Kellett GL. Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. Biochem J. 2000;350:149–54. [PMC free article] [PubMed] [Google Scholar]
- 40.Sugawara-Yokoo M, Suzuki T, Matsuzaki T, Naruse T, Takata K. Presence of fructose transporter GLUT5 in the S3 proximal tubules in the rat kidney. Kidney Int. 1999;56(3):1022–8. doi: 10.1046/j.1523-1755.1999.00635.x. [DOI] [PubMed] [Google Scholar]
- 41.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19(3):282–5. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 42.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol. 2005;67:411–43. doi: 10.1146/annurev.physiol.67.031103.153004. Review. [DOI] [PubMed] [Google Scholar]
- 43.Bookstein C, DePaoli AM, Xie Y, Niu P, Musch MW, Rao MC, Chang EB. Na+/H+ exchangers, NHE-1 and NHE-3, of rat intestine. Expression and localization. J Clin Invest. 1994;93(1):106–13. doi: 10.1172/JCI116933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl-/HCO3- exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol. 2002;282:G573–G579. doi: 10.1152/ajpgi.00338.2001. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz J, Seidler U, Aronson PS, Soleimani M. Kidney and intestine transport defects in Slc26a6 null mice. Am J Physiol Cell Physiol. 2005;288:C957–C965. doi: 10.1152/ajpcell.00505.2004. [DOI] [PubMed] [Google Scholar]
- 46.Simpson JE, Schweinfest C, Shull GE, Gawenis L, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the Predominant Apical Membrane Cl-/HCO3- Exchanger in the Upper Villous Epithelium of Murine Duodenum. Am J Physiol Gastrointest Liver Physiol. 2006 Dec 14; doi: 10.1152/ajpgi.00354.2006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Seidler U, Rottinghaus I, Hillesheim J, Chen M, Riederer B, Krabbenhöft A, Engelhardt R, Wiemann M, Wang Z, Barone S, Manns MP, Soleimani M. Sodium and chloride absorptive defects in the small intestine in Slc26a6 null mice. Pflugers Arch. 2008;455(4):757–66. doi: 10.1007/s00424-007-0318-z. [DOI] [PubMed] [Google Scholar]
- 48.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447:710–721. doi: 10.1007/s00424-003-1090-3. (Review) [DOI] [PubMed] [Google Scholar]
- 49.Soleimani M. Expression, regulation and the role of SLC26 Cl-/HCO3-exchangers in kidney and gastrointestinal tract. Novartis Found Symp. 2006;273:91–102. (Review) [PubMed] [Google Scholar]
- 50.Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol. 2003;283(4):F826–38. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]
- 51.Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci U S A. 2001;98:9425–9430. doi: 10.1073/pnas.141241098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrovic S, Ma L, Wang Z, Soleimani M. Identification of an apical Cl-/HCO-3 exchanger in rat kidney proximal tubule. Am J Physiol Cell Physiol. 2003;285:C608–C617. doi: 10.1152/ajpcell.00084.2003. [DOI] [PubMed] [Google Scholar]
- 53.Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg M-L, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhea. Nature Genet. 1996;14:316–319. doi: 10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- 54.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. Slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem. 2006;281(49):37962–71. doi: 10.1074/jbc.M607527200. [DOI] [PubMed] [Google Scholar]
- 55.Walker NM, Simpson JE, Yen PF, Gill RK, Rigsby EV, Brazill JM, Dudeja PK, Schweinfest CW, Clarke LL. Down-regulated in adenoma Cl/HCO3 exchanger couples with Na/H exchanger 3 for NaCl absorption in murine small intestine. Gastroenterology. 2008;135:1645–1653. doi: 10.1053/j.gastro.2008.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang T, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Giebisch G, Aronson PS. Mechanism of proximal tubule bicarbonate absorption in NHE3 null mice. Am J Physiol. 1999;277(2):F298–302. doi: 10.1152/ajprenal.1999.277.2.F298. [DOI] [PubMed] [Google Scholar]
- 57.Wang T, Hropot M, Aronson PS, Giebisch G. Role of NHE isoforms in mediating bicarbonate reabsorption along the nephron. Am J Physiol Renal Physiol. 2001;281(6):F1117–22. doi: 10.1152/ajprenal.2001.281.6.F1117. [DOI] [PubMed] [Google Scholar]
- 58.Barone S, Fussell SL, Singh AK, Lucas F, Xu J, Kim C, Wu X, Yu Y, Amlal H, Seidler U, Zuo J, Soleimani M. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J Biol Chem. 2009;20;284(8):5056–66. doi: 10.1074/jbc.M808128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Catena C, Cavarape A, Novello M, Giacchetti G, Sechi LA. Insulin receptors and renal sodium handling in hypertensive fructose-fed rats. Kidney Int. 2003;64(6):2163–71. doi: 10.1046/j.1523-1755.2003.00313.x. [DOI] [PubMed] [Google Scholar]