Abstract
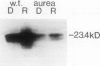
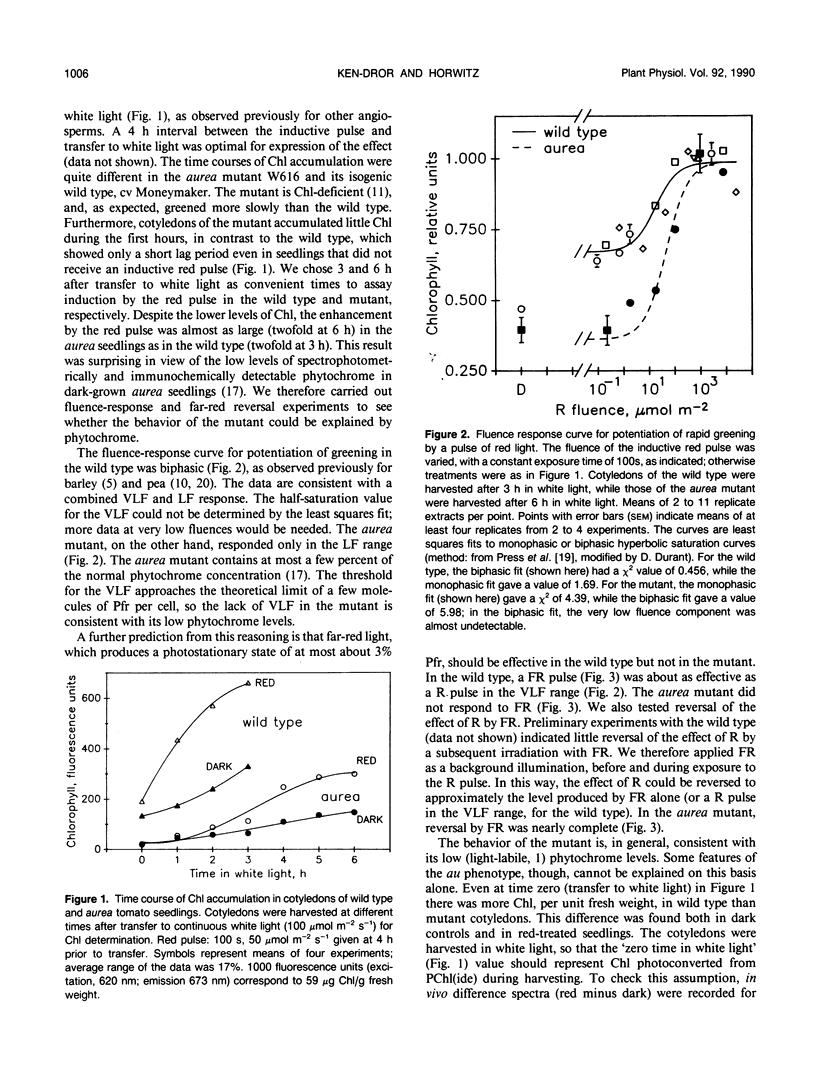
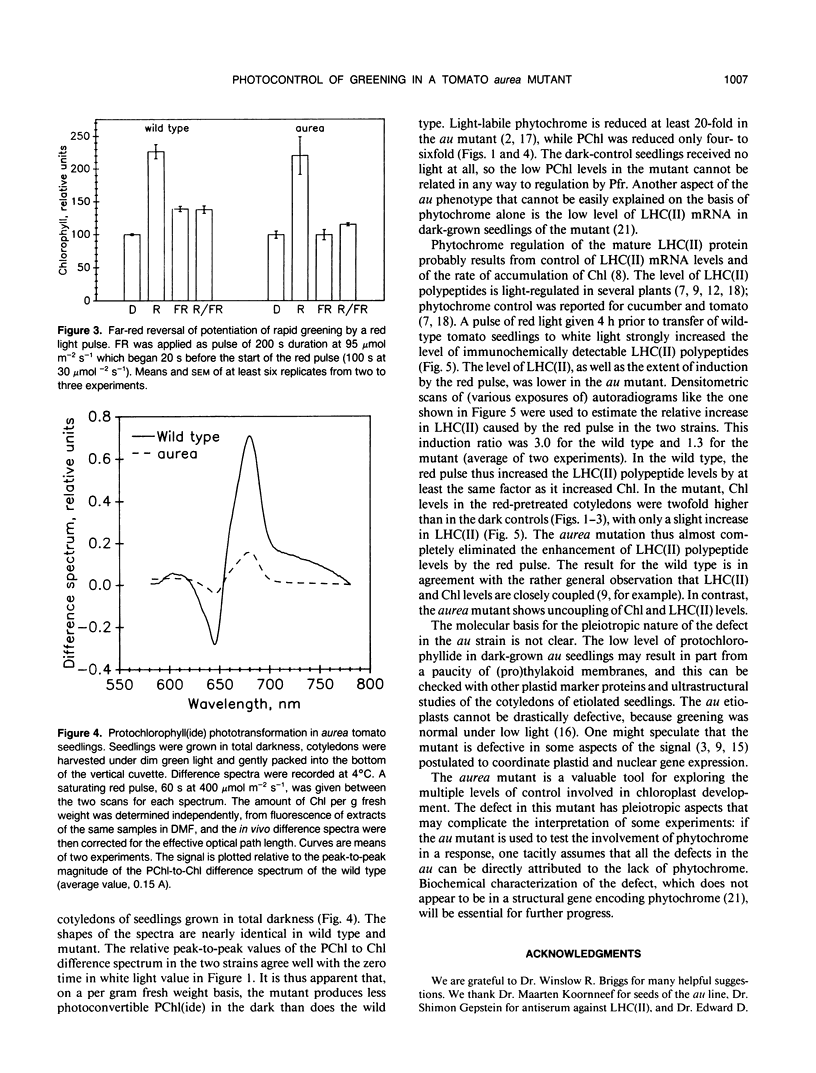
A brief pulse of red light accelerates chlorophyll accumulation upon subsequent transfer of dark-grown tomato (Lycopersicon esculentum) seedlings to continuous white light. Such potentiation of greening was compared in wild type and an aurea mutant W616. This mutant has been the subject of recent studies of phytochrome phototransduction; its dark-grown seedlings are deficient in phytochrome, and light-grown plants have yellow-green leaves. The rate of greening was slower in the mutant, but the extent (relative to the dark control) of potentiation by the red pulse was similar to that in the wild type. In the wild type, the fluence-response curve for potentiation of greening indicates substantial components in the VLF (very low fluence) and LF (low fluence) ranges. Far-red light could only partially reverse the effect of red. In the aurea mutant, only red light in the LF range was effective, and the effect of red was completely reversed by far-red light. When grown in total darkness, aurea seedlings are also deficient in photoconvertible PChl(ide). Upon transfer to white light, the aurea mutant was defective in both the abundance and light regulation of the light-harvesting chlorophyll a/b binding polypeptide(s) [LHC(II)]. The results are consistent with the VLF response in greening being mediated by phytochrome. Furthermore, the data support the hypothesis that light modulates LHC(II) levels through its control of the synthesis of both chlorophyll and its LHC(II) apoproteins. Some, but not all, aspects of the aurea phenotype can be accounted for by the deficiency in photoreception by phytochrome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batschauer A., Mösinger E., Kreuz K., Dörr I., Apel K. The implication of a plastid-derived factor in the transcriptional control of nuclear genes encoding the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1986 Feb 3;154(3):625–634. doi: 10.1111/j.1432-1033.1986.tb09444.x. [DOI] [PubMed] [Google Scholar]
- Briggs W. R., Mösinger E., Schäfer E. Phytochrome regulation of greening in barley-effects on chlorophyll accumulation. Plant Physiol. 1988 Feb;86(2):435–440. doi: 10.1104/pp.86.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L. Absorption spectroscopy of biological materials. Methods Enzymol. 1972;24:3–25. doi: 10.1016/0076-6879(72)24052-6. [DOI] [PubMed] [Google Scholar]
- Horwitz B. A., Thompson W. F., Briggs W. R. Phytochrome Regulation of Greening in Pisum: Chlorophyll Accumulation and Abundance of mRNA for the Light-Harvesting Chlorophyll a/b Binding Proteins. Plant Physiol. 1988 Jan;86(1):299–305. doi: 10.1104/pp.86.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liveanu V., Yocum C. F., Nelson N. Polypeptides of the oxygen-evolving photosystem II complex. Immunological detection and biogenesis. J Biol Chem. 1986 Apr 25;261(12):5296–5300. [PubMed] [Google Scholar]
- Moran R. Formulae for determination of chlorophyllous pigments extracted with n,n-dimethylformamide. Plant Physiol. 1982 Jun;69(6):1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelmüller R., Kendrick R. E., Briggs W. R. Blue-light mediated accumulation of nuclear-encoded transcripts coding for proteins of the thylakoid membrane is absent in the phytochrome-deficient aurea mutant of tomato. Plant Mol Biol. 1989 Aug;13(2):223–232. doi: 10.1007/BF00016140. [DOI] [PubMed] [Google Scholar]