Abstract
Aims:
We investigated the role of socioeconomic disparities in the association between diet and risk of type 2 diabetes (T2D).
Methods:
We used prospective data from 40,243 Sister Study participants aged 35 to 74 years who were enrolled in 2003–2009. Scores for healthy eating indices (alternate Mediterranean diet, Dietary Approaches to Stop Hypertension, alternative Healthy Eating Index, and Healthy Eating Index 2015 (HEI-2015)) were calculated using data from a 110-item food frequency questionnaire completed at enrollment. Incident T2D was defined based on self-reported physician’s diagnosis or use of anti-diabetic medications. Multivariable-adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards models.
Results:
We observed inverse associations between all four dietary indices and incident T2D after multivariable adjustment. These associations were most pronounced among women with higher educational attainment, higher income, and lower area deprivation index (ADI) (e.g., for the HEI-2015: low ADI, aHRQ4vsQ1: 0.44, 95% CI: 0.35, 0.56 vs high ADI, aHRQ4vsQ1: 0.75, 95% CI: 0.63, 0.90; pinteraction: 0.0007).
Conclusions:
Weaker associations among women with lower socioeconomic status and higher neighborhood deprivation suggests that other factors play a larger role in T2D incidence than diet quality among individuals with low SES.
Keywords: type 2 diabetes, dietary patterns, disparities, socioeconomic status, nutrition
INTRODUCTION
An estimated 32 million adults in the United States are living with diabetes [1]. By 2045, it is estimated that the prevalence of diabetes will increase by 16% [2]. Type 2 diabetes (T2D) accounts for 90% of diabetes cases and is associated with increased risk of neuropathy, nephropathy, and macrovascular complications [3].
Diet is a modifiable risk factor for T2D, and dietary patterns are commonly used to characterize overall diet quality in studies investigating the relationship between diet and T2D risk. Most evidence suggests that the alternate Mediterranean diet (aMED), Dietary Approaches to Stop Hypertension (DASH), and alternative Healthy Eating Index (aHEI) are associated with reduced risk of T2D [4–10]. However, evidence for the association between the Healthy Eating Index (HEI) and T2D risk is inconsistent [4–5,7–9,11]. In addition to diet, individual sociodemographic factors, such as income and education, are associated with T2D [12]. The incidence and prevalence of T2D are disproportionately higher among minoritized racial and ethnic groups and individuals with low socioeconomic status (SES) [12–13]. In addition, findings from several studies suggest that low neighborhood SES is associated with increased risk of T2D [14–15].
Though SES and diet quality have been extensively studied for their influence on the risk of T2D, few studies have investigated whether the association between diet quality and incident T2D risk differs by SES. In one previous study, this association was assessed using a single measure of diet quality (Lifelines Diet Score) across levels of educational attainment, the sole indicator of SES [16]. To our knowledge, no studies have examined whether neighborhood-level SES indicators, such as the area deprivation index (ADI), modify the relationship between dietary patterns and incident T2D. A deeper understanding of the interactions between diet and SES and their multifaceted roles in T2D risk could have implications for public health policy and T2D prevention. The purpose of this study is to examine the relationship of established dietary indices (aMED, DASH, aHEI, and HEI-2015) with incident T2D and investigate whether this relationship is modified by individual and neighborhood SES indicators.
MATERIALS AND METHODS
Study Population
Data were obtained from the Sister Study, a prospective cohort study designed to identify environmental and genetic factors for breast cancer [17]. The Sister Study consists of 50,884 self-identified women in the United States, including Puerto Rico, enrolled between 2003 and 2009. Women were eligible for enrollment if they were aged 35–74 years and had a sister who had been diagnosed with breast cancer but had no personal history of a breast cancer diagnosis themselves.
At baseline, data on demographic, medical, lifestyle, and reproductive factors were collected using computer-assisted telephone interviews and self-completed questionnaires. Anthropometric measurements and biological samples were collected during a home exam. Participants provided annual health updates and completed detailed follow-up questionnaires every 2–3 years. Response rates have been around 90% throughout follow-up [17]. The Sister Study is overseen by the National Institutes of Health Institutional Review Board. All participants provided written informed consent.
Dietary Assessment
Diet was assessed at baseline using a modified 1998 Block 110-item food frequency questionnaire (FFQ) previously validated in women [18–19]. Participants reported average dietary intake in the past 12 months of each listed food and beverage item, including frequency of intake (9 possible frequencies ranging from “never” to “every day”) and portion size (3 or 4 quantity choices per food item or group of similar food items). Nutrient intake was estimated using the United States Department of Agriculture (USDA) Food and Nutrient Database for Dietary Studies (FNDDS) for U.S. women [20].
The Healthy Eating Index (HEI), originally developed to examine the extent to which diet aligns with the Dietary Guidelines for Americans, is updated every five years with dietary recommendations for Americans [21]. HEI-2015 reflects the guidelines for 2015–2020. We used the previously developed aMED, modified and adapted from the Mediterranean diet (MED) characterized by high intake of plant-based foods, olive oil, and minimal consumption of saturated and trans fats, meats, and dairy products [22–23]. aMED scores were operationalized using the method developed by Fung et al [23]. The DASH diet is characterized by higher intake of fruits, vegetables, legumes, and nuts and minimal intake of sugary foods, sodium, and animal products [24]. aHEI is based on foods and nutrients that predict chronic disease risk [8]. Additional details of these dietary patterns, including how the scores are calculated, are provided in Supplementary Tables 1a and 1b.
Area Deprivation Index
ADI was used to characterize neighborhood deprivation of residence at enrollment. Seventeen weighted US census indicators of educational attainment, income, employment, and housing (e.g., unemployment rate and median home value) were obtained from the U.S. Census and the American Community Survey and used to construct the 2000 ADI [25–26]. Each neighborhood received a percentile ranking, with higher percentage of ADI corresponding to greater neighborhood deprivation. Census block-group identification codes were used to link each participants’ enrollment residential address and the ADI. Participants were categorized into three groups based on ADI tertiles within the study sample.
Identification of T2D
Annual follow-up questionnaires were used to ascertain incident T2D. Follow-up was through September 2019 (data release 9.1, median 11.6 years of follow-up). Participants were asked, “Has a doctor or other healthcare provider ever told you that you had diabetes?” Women who responded “Yes” or who self-reported use of oral anti-diabetic medication or insulin use were assumed to have incident T2D. To identify incident T2D, women were excluded at baseline if they had likely type 1 diabetes (n=141), a prior history of secondary diabetes (n=24), or prevalent T2D (n=3,836). Women were classified as type 1 diabetes if they: 1) self-reported T1D, 2) were diagnosed with diabetes prior to 20 years old, or 3) received a diabetes diagnosis between the ages of 20 and 34 and began taking insulin less than 12 months following diagnosis [27]. Women with diabetes were classified as having secondary diabetes if they also had hemochromatosis, hepatitis, drug-induced diabetes, liver cirrhosis, hyperthyroidism, polycystic ovary syndrome, or gestational diabetes within 12 months prior to T2D diagnosis [28]. Women taking anti-diabetic medication at enrollment or within the past 12 months or who self-reported being told by a physician or healthcare provider they had non-pregnancy related T2D at baseline were assumed to have prevalent T2D.
Covariate Assessment
Covariates were assessed through questionnaires at baseline. Details on the categorization of covariates are provided in Table 1 and Supplementary Material.
Table 1.
Characteristics of Sister Study participants at baseline by HEI-2015 quartiles
| Characteristics | Q1 (N=10059) | Q2 (N=10061) | Q3 (N=10061) | Q4 (N=10062) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||
| Age at baseline, years | 52.5 (8.7) | 54.4 (8.8) | 55.9 (8.7) | 57.9 (8.6) | ||||
| BMI at baseline, kg/m 2 | 28.5 (6.2) | 27.6 (5.7) | 26.8 (5.3) | 25.7 (4.9) | ||||
| Total energy intake, kcal/day | 1664.7 (668.1) | 1623.6 (616.1) | 1622.5 (577.6) | 1629.8 (525.3) | ||||
| N | % | N | % | N | % | N | % | |
| Race and Ethnicity * | ||||||||
| Non-Hispanic White | 8585 | 85.4 | 8705 | 86.5 | 8820 | 87.7 | 9008 | 89.5 |
| Non-Hispanic Black | 804 | 8.0 | 809 | 8.0 | 738 | 7.3 | 598 | 5.9 |
| Other | 670 | 6.7 | 547 | 5.4 | 503 | 5.0 | 456 | 4.5 |
| Educational Attainment | ||||||||
| ≤ High school diploma | 2040 | 20.3 | 1428 | 14.2 | 1165 | 11.6 | 1086 | 10.8 |
| Some college | 3885 | 38.6 | 3397 | 33.8 | 3182 | 31.6 | 2797 | 27.8 |
| ≥ College degree | 4134 | 41.1 | 5236 | 52.0 | 5714 | 56.8 | 6179 | 61.4 |
| Household Income | ||||||||
| <$50,000 | 2684 | 26.7 | 2157 | 21.4 | 2073 | 20.6 | 2218 | 22.0 |
| $50,000-$99,999 | 4301 | 42.8 | 4246 | 42.2 | 4133 | 41.1 | 4063 | 40.4 |
| ≥ 100,000 | 3074 | 30.6 | 3658 | 36.4 | 3855 | 38.3 | 3781 | 37.6 |
| ADI | ||||||||
| ≤ 17 | 2581 | 25.7 | 3243 | 32.2 | 3497 | 34.8 | 3754 | 37.3 |
| 18–39 | 3119 | 31.0 | 3235 | 32.1 | 3190 | 31.7 | 3258 | 32.4 |
| > 39 | 4359 | 43.3 | 3583 | 35.6 | 3374 | 33.5 | 3050 | 30.3 |
| Smoking Status | ||||||||
| Never | 5209 | 51.8 | 5690 | 56.6 | 5845 | 58.1 | 6079 | 60.4 |
| > 0 and < 10 pack-years | 1973 | 19.6 | 2240 | 22.3 | 2325 | 23.1 | 2398 | 23.8 |
| ≥ 10 and < 20 pack-years | 1051 | 10.5 | 948 | 9.4 | 895 | 8.9 | 777 | 7.7 |
| ≥ 20 pack-years | 1826 | 18.2 | 1183 | 11.8 | 996 | 9.9 | 808 | 8.0 |
| Alcohol Consumption | ||||||||
| Never | 310 | 3.1 | 294 | 2.9 | 305 | 3.0 | 357 | 3.6 |
| Former | 1661 | 16.5 | 1325 | 13.2 | 1157 | 11.5 | 1323 | 13.2 |
| Current drinker, <1 drink/day | 6770 | 67.3 | 6885 | 68.4 | 7005 | 69.6 | 6976 | 69.3 |
| Current drinker, 1–1.9 drink/day | 812 | 8.1 | 984 | 9.8 | 1047 | 10.4 | 941 | 9.4 |
| Current drinker, ≥ 2 drink/day | 506 | 5.0 | 573 | 5.7 | 547 | 5.4 | 465 | 4.6 |
| Hormone Therapy Use | ||||||||
| None | 6596 | 65.6 | 6079 | 60.4 | 5673 | 56.4 | 5082 | 50.5 |
| Estrogen only | 1780 | 17.7 | 1817 | 18.1 | 1945 | 19.3 | 2077 | 20.6 |
| Progesterone or combination therapy | 1683 | 16.7 | 2165 | 21.5 | 2443 | 24.3 | 2903 | 28.9 |
| Hormonal Birth Control Use | ||||||||
| No | 1185 | 11.8 | 1277 | 12.7 | 1336 | 13.3 | 1740 | 17.3 |
| Yes | 8874 | 88.2 | 8784 | 87.3 | 8725 | 86.7 | 8322 | 82.7 |
| Menopausal Status | ||||||||
| Premenopausal | 4590 | 45.6 | 3893 | 38.7 | 3274 | 32.5 | 2495 | 24.8 |
| Postmenopausal | 5469 | 54.4 | 6168 | 61.3 | 6787 | 67.5 | 7567 | 75.2 |
| High Cholesterol | ||||||||
| No | 8355 | 83.1 | 8260 | 82.1 | 8093 | 80.4 | 8011 | 79.6 |
| Yes | 1704 | 16.9 | 1801 | 17.9 | 1968 | 19.6 | 2051 | 20.4 |
| Hypertension | ||||||||
| No hypertension | 4704 | 46.8 | 4917 | 48.9 | 4886 | 48.6 | 5205 | 51.7 |
| Pre-hypertensive | 752 | 7.5 | 712 | 7.1 | 781 | 7.8 | 719 | 7.2 |
| Hypertensive | 4603 | 45.8 | 4432 | 44.0 | 4394 | 43.7 | 4138 | 41.1 |
| Vitamin/Supplement Use | ||||||||
| None | 1144 | 11.4 | 937 | 9.3 | 779 | 7.7 | 668 | 6.6 |
| Few days/month or 1–3 days/week | 1170 | 11.6 | 1271 | 12.6 | 1128 | 11.2 | 867 | 8.6 |
| 4–6 days per week | 1116 | 11.1 | 1373 | 13.7 | 1489 | 14.8 | 1380 | 13.7 |
| Everyday | 3131 | 31.1 | 3939 | 39.2 | 4670 | 46.4 | 5656 | 56.2 |
| Missing | 3498 | 34.8 | 2541 | 25.3 | 1995 | 19.8 | 1491 | 14.8 |
| Family History of Type 2 Diabetes | ||||||||
| No | 3789 | 37.7 | 4058 | 40.3 | 4032 | 40.1 | 4214 | 41.9 |
| Yes | 5336 | 53.1 | 5198 | 51.7 | 5248 | 52.2 | 5103 | 50.7 |
| Missing | 934 | 9.3 | 805 | 8.0 | 781 | 7.8 | 745 | 7.4 |
| Quintiles of MET-hours of physical activity/week | ||||||||
| ≤ 24 | 9022 | 89.7 | 8339 | 82.9 | 7827 | 77.8 | 7056 | 70.1 |
| 25–38 | 615 | 6.1 | 967 | 9.6 | 1194 | 11.9 | 1555 | 15.5 |
| 39–53 | 252 | 2.5 | 418 | 4.2 | 557 | 5.5 | 809 | 8.0 |
| 53–75 | 121 | 1.2 | 233 | 2.3 | 330 | 3.3 | 414 | 4.1 |
| > 75 | 49 | 0.5 | 104 | 1.0 | 153 | 1.5 | 228 | 2.3 |
Abbreviations: HEI-2015, Healthy Eating Index 2015; BMI, body mass index; ADI, area deprivation index, type 2 diabetes; MET, metabolic equivalent
Quartile ranges: Q1: ≤ 65.75568, Q2: 65.75569–72.8042, Q3: 72.8043–79.0720, Q4: > 79.0720
Women in the “Other” race and ethnicity category identified as non-Hispanic for ethnicity and Native Hawaiian or other Pacific Islander, Asian, American Indian or Alaskan Native, or had no specified race
Statistical Analysis
In addition to exclusions described above, women with a missing date of T2D diagnosis (n=574) were excluded. Person-time within the first 12 months of follow-up (n=461) was excluded to reduce bias related to undetected T2D present at baseline. Additionally, women with missing dietary data (n=1,335), missing covariate data (n=4,106), except for vitamin/supplement use and family history of T2D, or BMI less than 15 kg/m2 or greater than 50 kg/m2 (n=164) were excluded. Implausible energy intake (<500 or >5000 kcals/day) was considered as an exclusion criterion, but after applying previous exclusion criteria, no observations in the study sample had implausible energy intake. The final study sample consisted of 40,243 women.
Frequencies and proportions are reported for categorical variables and means and standard deviations are reported for continuous variables. Based on Schoenfeld residuals, the Cox proportional hazards assumption was satisfied. Multivariable-adjusted hazard ratios (HRs) and 95% CIs were estimated using Cox proportional hazards models with age as the primary time scale. Dietary patterns were expressed in quartiles based on the distribution in the study sample and as continuous measures. Statistical models were adjusted for baseline measures of total energy intake, race and ethnicity, educational attainment, household income, smoking status, vitamin/supplement use in the past 12 months, ever use of hormone contraceptives, hormone therapy, family history of T2D, quintiles of total MET-hours of physical activity per week, and ADI. DASH and HEI-2015 models were additionally adjusted for alcohol consumption. aMED and aHEI contain alcohol as a component of the score, and thus, alcohol was not included in the multivariable models for these dietary patterns. Body mass index (BMI), hypertension, and hypercholesterolemia are potential mediators of the association between dietary patterns and risk of T2D and thus, were not included in the multivariable-adjusted models. However, as part of sensitivity analysis these covariates were added to the regression models. Linear tests for trend were calculated by modeling the quartiles of dietary indices as ordinal. A two-sided p value of less than 0.05 was considered statistically significant in the main effect models.
Effect modification by individual sociodemographic characteristics (race and ethnicity, education, and income) and by neighborhood-level ADI was assessed by stratification. Joint associations of ADI and HEI-2015 were examined using a common referent group (low ADI and high HEI-2015) and comparing all other combinations to the common referent group. The relative excess risk due to interaction (RERI) was calculated to assess interaction on the additive scale [29–30]. Interaction p-values were determined by including an interaction term in the adjusted models. P values less than 0.10 were considered statistically significant in interaction models. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Table 1 shows characteristics of Sister Study participants at baseline by HEI-2015 quartiles. We focus on HEI-2015 because it was the only dietary pattern in which the interaction with all three SES variables (education, income, and ADI) were statistically significant. Descriptive statistics by quartiles of the other three dietary patterns are provided in Supplementary Tables 2-4. Women in the highest quartile of HEI-2015 were more likely to be older, have lower BMI, be non-Hispanic White (NHW), have a college degree, be never smokers, be postmenopausal, and be less sedentary compared to women in the lowest quartile. Descriptive characteristics were similarly distributed across quartiles of the other dietary patterns (Supplementary Tables 2-4). Women living in the most deprived neighborhoods tended to have higher BMI, lower educational attainment, lower income, and be smokers of ≥20 pack-years as compared to those in less deprived areas (Supplementary Table 5). Median HEI-2015 scores were highest among women with at least a college degree, an income greater than $100,000, and the lowest neighborhood deprivation (Supplementary Table 6).
During 408,756 person-years of follow up, 2,486 women developed T2D. HRs and 95% CIs for the association between incident T2D and dietary pattern scores are presented in Table 2. Women in the highest HEI-2015, aMED, DASH, and aHEI quartiles had a 37%, 34%, 43%, and 42% reduction in the hazard of T2D, respectively, compared to women in the lowest quartiles (aHRHEI-2015: 0.63, 95% CI: 0.55, 0.71; aHRaMED: 0.66, 95% CI: 0.58, 0.75; aHRDASH: 0.57, 95% CI: 0.51, 0.65; aHRaHEI: 0.58, 95% CI: 0.51, 0.65), and there was evidence of decreasing trend in T2D risk with increasing dietary pattern scores (all Ptrend <0.0001). In addition, each one SD increase in all dietary indices was inversely associated with T2D risk. In a sensitivity analysis that included additional adjustment for BMI, hypertension, and high cholesterol (Supplementary Table 7), results were attenuated but generally consistent with those in Table 2. In the aMED model, there was no significant evidence of a linear trend.
Table 2:
Hazard Ratios (HR) and 95% confidence intervals (CIs) for the associations of dietary pattern scores and socioeconomic indictors with incident type 2 diabetes
| Dietary Patterns | ||||
|---|---|---|---|---|
| Cases/person-years | HR | 95% CI | Ptrend | |
| HEI-2015 a | <.0001 | |||
| Q1 | 823/99743 | 1.00 | Ref | |
| Q2 | 643/102040 | 0.84 | 0.75, 0.93 | |
| Q3 | 553/102928 | 0.74 | 0.66, 0.82 | |
| Q4 | 467/104044 | 0.63 | 0.55, 0.71 | |
| Continuous | 2486/408756 | 0.83 | 0.80, 0.87 | |
| aMED b | <.0001 | |||
| Q1 | 617/86458 | 1.00 | Ref | |
| Q2 | 899/133928 | 0.93 | 0.84, 1.03 | |
| Q3 | 399/68607 | 0.80 | 0.70, 0.92 | |
| Q4 | 571/119763 | 0.66 | 0.58, 0.75 | |
| Continuous | 2486/408756 | 0.84 | 0.80, 0.88 | |
| DASH a | <.0001 | |||
| Q1 | 819/93290 | 1.00 | Ref | |
| Q2 | 718/115123 | 0.76 | 0.69, 0.84 | |
| Q3 | 469/90833 | 0.65 | 0.58, 0.73 | |
| Q4 | 480/109509 | 0.57 | 0.51, 0.65 | |
| Continuous | 2486/408756 | 0.80 | 0.76, 0.83 | |
| aHEI b | <.0001 | |||
| Q1 | 824/99386 | 1.00 | Ref | |
| Q2 | 679/101546 | 0.87 | 0.79, 0.97 | |
| Q3 | 571/103571 | 0.75 | 0.67, 0.83 | |
| Q4 | 412/104253 | 0.58 | 0.51, 0.65 | |
| Continuous | 2486/408756 | 0.81 | 0.77, 0.84 | |
| SES Indicators | ||||
| Cases/person-years | HR | 95% CI | ||
| ADI c | <.0001 | |||
| ≤ 17 | 587/135893 | 1.00 | ref | |
| 18–39 | 769/131237 | 1.15 | 1.03, 1.28 | |
| > 39 | 1130/141626 | 1.31 | 1.18, 1.46 | |
| Educational Attainment c | 0.002 | |||
| ≤ High school diploma | 434/55271 | 1.00 | ref | |
| Some college | 946/130949 | 1.03 | 0.91, 1.15 | |
| ≥ College degree | 1106/222536 | 0.87 | 0.77, 0.98 | |
| Household Income c | <.0001 | |||
| <$50,000 | 775/88477 | 1.00 | ref | |
| $50,000-$99,999 | 1100/170631 | 0.88 | 0.80, 0.97 | |
| ≥ 100,000 | 611/149648 | 0.67 | 0.60, 0.76 | |
Abbreviations: ADI, area deprivation index; aHEI, alternative Healthy Eating Index; aMED, alternate Mediterranean Diet; DASH, Dietary Approaches to Stop Hypertension; HEI-2015, Healthy Eating Index 2015; SES, socioeconomic status
Quartile ranges:
HEI-2015: Q1: ≤ 65.75568, Q2: 65.75569–72.8042, Q3: 72.8043–79.0720, Q4: > 79.0720 aMED: Q1: ≤ 2, Q2: 3–4, Q3: 5, Q4: > 5
DASH: Q1: ≤ 20, Q2: 21–24, Q3: 25–27, Q4: > 27
aHEI: Q1: ≤ 52.07, Q2: 52.08–60.44, Q3: 60.45–68.81, Q4: > 68.81
Adjusted for race, income, education, menopausal status, smoking status, alcohol consumption, energy intake, physical activity, vitamin/supplement use, hormone therapy, hormonal contraceptive use, family history of type 2 diabetes, and ADI
Adjusted for all variables in a with the exception of alcohol consumption
Adjusted for all variables in a, and mutually adjusted for income, education, and ADI.
Age is the primary time scale, and thus, not included as a covariate in the multivariable models.
Estimates for the associations between socioeconomic indicators and T2D risk are also shown in Table 2. Women living in areas with high neighborhood deprivation had a higher risk of T2D compared to women with low neighborhood deprivation (HR: 1.31, 95% CI: 1.18, 1.46). Comparing those in the highest to lowest education category, the risk of T2D was lower among women with at least a college degree (HR: 0.87, 95% CI: 0.77, 0.98). Compared to women with income <$50,000/year, the risk of T2D was reduced among women reporting income of ≥$100,000/year (HR: 0.67, 95% CI: 0.60, 0.76). Following adjustment for BMI, hypertension, and high cholesterol (Supplementary Table 7), the association between ADI and T2D risk was attenuated, educational attainment was no longer associated with T2D risk, and the HR in the household income model was slightly attenuated.
Associations between dietary patterns and T2D stratified by race and ethnicity, education, income, and ADI are shown in Table 3. In models stratified by race and ethnicity comparing the highest quartile to the lowest quartile of dietary pattern scores, associations with T2D were similar for NHW, non-Hispanic Black (NHB), and other race and ethnicity groups except for the HEI-2015 where the association was null among NHB women. The inverse relationship between HEI-2015 and T2D risk was strongest for women with at least a college degree (aHRQ4vsQ1: 0.56, 95% CI: 0.46, 0.66). Comparing the highest to lowest HEI-2015 quartile, risk estimates were attenuated among women with a household income of less than $50,000/year. Within strata of ADI, comparing the highest quartile of HEI-2015 to the lowest, the risk of T2D was lowest among women with low neighborhood deprivation (low ADI aHRQ4vsQ1: 0.44, 95% CI: 0.35, 0.56; high ADI aHRQ4vsQ1: 0.75, 95% CI: 0.63, 0.90; pinteraction: 0.0007). Similar stratified risk estimates were observed in aMED, DASH, and aHEI models. In contrast to the other dietary patterns, there was evidence of statistical interaction between aMED and race and ethnicity (pinteraction: 0.02), with a stronger association observed among women who were neither NHW nor NHB.
Table 3.
Associations between dietary pattern scores and incident type 2 diabetes stratified by race, education, income, and ADI
| Quartiles of HEI-2015a | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Pinteraction | |
| Race and Ethnicity | 0.24 | ||||
| Non-Hispanic White | 1.00 (ref) | 0.83 (0.74, 0.94) | 0.71 (0.62, 0.81) | 0.61 (0.53, 0.70) | |
| Non-Hispanic Black | 1.00 (ref) | 0.82 (0.63, 1.09) | 0.76 (0.57, 1.02) | 0.82 (0.59, 1.13) | |
| Other | 1.00 (ref) | 0.84 (0.58, 1.22) | 0.93 (0.64, 1.35) | 0.51 (0.32, 0.82) | |
| Educational Attainment | 0.07 | ||||
| ≤ High school diploma | 1.00 (ref) | 0.80 (0.62, 1.03) | 0.79 (0.60, 1.03) | 0.77 (0.58, 1.02) | |
| Some college | 1.00 (ref) | 0.86 (0.73, 1.01) | 0.78 (0.65, 0.93) | 0.68 (0.55, 0.83) | |
| ≥ College degree | 1.00 (ref) | 0.83 (0.70, 0.98) | 0.68 (0.58, 0.81) | 0.56 (0.46, 0.66) | |
| Household Income | 0.06 | ||||
| <$50,000 | 1.00 (ref) | 0.86 (0.71, 1.05) | 0.94 (0.77, 1.14) | 0.78 (0.63, 0.96) | |
| $50,000-$99,999 | 1.00 (ref) | 0.87 (0.75, 1.02) | 0.71 (0.60, 0.84) | 0.57 (0.47, 0.69) | |
| ≥ 100,000 | 1.00 (ref) | 0.74 (0.60, 0.92) | 0.57 (0.46, 0.72) | 0.56 (0.44, 0.70) | |
| ADI | 0.0007 | ||||
| ≤ 17 | 1.00 (ref) | 0.68 (0.55, 0.84) | 0.53 (0.42, 0.67) | 0.44 (0.35, 0.56) | |
| 18–39 | 1.00 (ref) | 0.75 (0.62, 0.91) | 0.71 (0.58, 0.87) | 0.61 (0.49, 0.76) | |
| > 39 | 1.00 (ref) | 0.98 (0.84, 1.14) | 0.87 (0.74, 1.02) | 0.75 (0.63, 0.90) | |
| Quartiles of aMEDb | |||||
| Q1 | Q2 | Q3 | Q4 | Pinteraction | |
| Race and Ethnicity | 0.02 | ||||
| Non-Hispanic White | 1.00 (ref) | 0.96 (0.85, 1.08) | 0.76 (0.65, 0.88) | 0.67 (0.57, 0.77) | |
| Non-Hispanic Black | 1.00 (ref) | 0.84 (0.64, 1.12) | 0.87 (0.61, 1.23) | 0.68 (0.49, 0.96) | |
| Other | 1.00 (ref) | 0.75 (0.51, 1.09) | 1.02 (0.67, 1.54) | 0.50 (0.31, 0.78) | |
| Educational Attainment | 0.01 | ||||
| ≤ High school diploma | 1.00 (ref) | 1.01 (0.80, 1.27) | 0.83 (0.60, 1.16) | 0.93 (0.68, 1.25) | |
| Some college | 1.00 (ref) | 0.93 (0.79, 1.09) | 0.80 (0.64, 0.98) | 0.69 (0.56, 0.85) | |
| ≥ College degree | 1.00 (ref) | 0.88 (0.74, 1.05) | 0.77 (0.63, 0.94) | 0.57 (0.47, 0.69) | |
| Household Income | 0.32 | ||||
| <$50,000 | 1.00 (ref) | 1.09 (0.91, 1.31) | 0.97 (0.76, 1.24) | 0.89 (0.71, 1.13) | |
| $50,000-$99,999 | 1.00 (ref) | 0.86 (0.74, 1.01) | 0.73 (0.60, 0.89) | 0.59 (0.48, 0.71) | |
| ≥ 100,000 | 1.00 (ref) | 0.87 (0.70, 1.09) | 0.74 (0.56, 0.96) | 0.54 (0.42, 0.71) | |
| ADI | 0.02 | ||||
| ≤ 17 | 1.00 (ref) | 0.79 (0.63, 0.99) | 0.76 (0.58, 0.98) | 0.42 (0.32, 0.55) | |
| 18–39 | 1.00 (ref) | 0.92(0.76, 1.12) | 0.73 (0.57, 0.93) | 0.74 (0.59, 0.94) | |
| > 39 | 1.00 (ref) | 1.00 (0.86, 1.16) | 0.85 (0.69, 1.03) | 0.75 (0.62, 0.91) | |
| Quartiles of DASHa | |||||
| Q1 | Q2 | Q3 | Q4 | Pinteraction | |
| Race and Ethnicity | 0.44 | ||||
| Non-Hispanic White | 1.00 (ref) | 0.75 (0.67, 0.85) | 0.64 (0.56, 0.73) | 0.59 (0.52, 0.68) | |
| Non-Hispanic Black | 1.00 (ref) | 0.84 (0.65, 1.08) | 0.75 (0.55, 1.03) | 0.57 (0.39, 0.83) | |
| Other | 1.00 (ref) | 0.79 (0.56 1.12) | 0.75 (0.49, 1.15) | 0.53 (0.33, 0.85) | |
| Educational Attainment | 0.03 | ||||
| ≤ High school diploma | 1.00 (ref) | 0.87 (0.69, 1.09) | 0.63 (0.47, 0.85) | 0.70 (0.52, 0.94) | |
| Some college | 1.00 (ref) | 0.74 (0.63, 0.87) | 0.70 (0.58, 0.85) | 0.65 (0.53, 0.79) | |
| ≥ College degree | 1.00 (ref) | 0.74 (0.63, 0.87) | 0.61 (0.51, 0.73) | 0.50 (0.42, 0.60) | |
| Household Income | 0.70 | ||||
| <$50,000 | 1.00 (ref) | 0.80 (0.66, 0.96) | 0.77 (0.62, 0.95) | 0.72 (0.58, 0.90) | |
| $50,000-$99,999 | 1.00 (ref) | 0.78 (0.67, 0.90) | 0.58 (0.49, 0.70) | 0.51 (0.42, 0.61) | |
| ≥ 100,000 | 1.00 (ref) | 0.70 (0.57, 0.87) | 0.64 (0.51, 0.81) | 0.53 (0.42, 0.67) | |
| ADI | 0.14 | ||||
| ≤ 17 | 1.00 (ref) | 0.65 (0.52, 0.82) | 0.58 (0.45, 0.73) | 0.47 (0.37, 0.59) | |
| 18–39 | 1.00 (ref) | 0.76 (0.63, 0.91) | 0.61 (0.49, 0.75) | 0.56 (0.45, 0.70) | |
| > 39 | 1.00 (ref) | 0.81 (0.70, 0.94) | 0.72 (0.60, 0.85) | 0.64 (0.53, 0.77) | |
| Quartiles of aHEIb | |||||
| Q1 | Q2 | Q3 | Q4 | Pinteraction | |
| Race and Ethnicity | 0.54 | ||||
| Non-Hispanic White | 1.00 (ref) | 0.85 (0.76, 0.96) | 0.77 (0.68, 0.87) | 0.59 (0.51, 0.68) | |
| Non-Hispanic Black | 1.00 (ref) | 0.96 (0.74, 1.25) | 0.68 (0.49, 0.93) | 0.62 (0.43, 0.90) | |
| Other | 1.00 (ref) | 0.99 (0.69, 1.43) | 0.82 (0.56, 1.21) | 0.55 (0.33, 0.92) | |
| Educational Attainment | 0.17 | ||||
| ≤ High school diploma | 1.00 (ref) | 0.93 (0.73, 1.17) | 0.88 (0.68, 1.13) | 0.69 (0.49, 0.97) | |
| Some college | 1.00 (ref) | 0.89 (0.76, 1.05) | 0.75 (0.63, 0.90) | 0.57 (0.46, 0.70) | |
| ≥ College degree | 1.00 (ref) | 0.81 (0.69, 0.96) | 0.70 (0.59, 0.82) | 0.55 (0.46, 0.65) | |
| Household Income | 0.13 | ||||
| <$50,000 | 1.00 (ref) | 0.85 (0.71,1.03) | 0.87 (0.71, 1.05) | 0.73 (0.58, 0.92) | |
| $50,000-$99,999 | 1.00 (ref) | 0.85 (0.72, 0.99) | 0.70 (0.59, 0.82) | 0.53 (0.44, 0.64) | |
| ≥ 100,000 | 1.00 (ref) | 0.96 (0.77, 1.19) | 0.73 (0.58, 0.92) | 0.53 (0.41, 0.68) | |
| ADI | 0.37 | ||||
| ≤ 17 | 1.00 (ref) | 0.78 (0.62, 0.98) | 0.65 (0.52, 0.82) | 0.48 (0.38, 0.61) | |
| 18–39 | 1.00 (ref) | 0.85 (0.71, 1.03) | 0.78 (0.64, 0.94) | 0.57 (0.46, 0.72) | |
| > 39 | 1.00 (ref) | 0.91 (0.79, 1.06) | 0.76 (0.65, 0.90) | 0.64 (0.52, 0.78) | |
Abbreviations: ADI, area deprivation index; aHEI, alternative Healthy Eating Index; aMED, alternate Mediterranean Diet; DASH, Dietary Approaches to Stop Hypertension; HEI-2015, Healthy Eating Index 2015
Quartile ranges:
HEI-2015: Q1: ≤ 65.75568, Q2: 65.75569–72.8042, Q3: 72.8043–79.0720, Q4: > 79.0720
aMED: Q1: ≤ 2, Q2: 3–4, Q3: 5, Q4: > 5
DASH: Q1: ≤ 20, Q2: 21–24, Q3: 25–27, Q4: > 27
aHEI: Q1: ≤ 52.07, Q2: 52.08–60.44, Q3: 60.45–68.81, Q4: > 68.81
Adjusted for menopausal status, smoking status, alcohol consumption, energy intake, physical activity, vitamin/supplement use, hormone therapy, hormonal contraceptive use, family history of type 2 diabetes, and mutually adjusted for race, income, education, and ADI
Adjusted for all variables in a with the exception of alcohol consumption
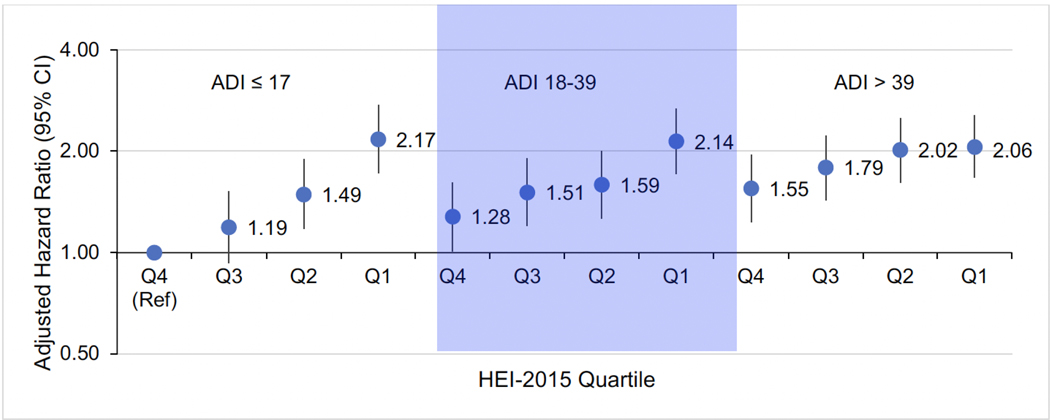
The joint associations of HEI-2015 and ADI with the highest HEI-2015 quartile and lowest ADI tertile as the common referent are presented in Figure 1. Among women with high neighborhood deprivation (ADI > 39) in the lowest HEI-2015 quartile, the risk of T2D increased by 2-fold (HR: 2.06, 95% CI: 1.67, 2.56). Similar risk estimates were observed among women with moderate (ADI 18–39) and low (ADI ≤ 17) neighborhood deprivation in the lowest HEI-2015 quartile. Among women with high neighborhood deprivation who were in the highest category of diet quality, the increased risk of T2D was attenuated (HR: 1.55, 95% CI: 1.23, 1.95). Among women with high neighborhood deprivation (ADI > 39) in HEI-2015 quartile 1, RERI was −0.66, indicating negative interaction on the additive scale (RERI: −0.66, 95% CI: −1.18, −0.14) (Supplementary Table 8).
Figure 1.
Plot of the joint associations of HEI-2015 and ADI with type 2 diabetes incidence.
HEI-2015 quartile ranges: Q1: ≤ 65.75568, Q2: 65.75569–72.8042, Q3: 72.8043–79.0720, Q4: > 79.0720
Compared to women who were included in the analyses, those who were excluded tended to be older, with higher BMI and ADI, and lower physical activity levels (Supplementary Table 9). A higher proportion of excluded participants were NHB or Other race and ethnicity, lower income, former or never drinkers, postmenopausal, and had high cholesterol, hypertension, and a family history of T2D.
DISCUSSION
In this prospective cohort study of women with a family history of breast cancer, higher scores on four dietary indices representing a better-quality diet were inversely associated with incident T2D. Inverse associations were highest in magnitude among women with higher individual SES and lower neighborhood deprivation, whereas the associations were less pronounced among those with lower individual SES and higher neighborhood deprivation.
Studies investigating the relationship between HEI and incident T2D have produced inconsistent results. Most studies have reported null findings, while other studies, including ours, observed significant inverse associations [4–5,7–9,11]. HEI assesses conformity to the Dietary Guidelines for Americans and is updated every 5 years [21]. Since studies using HEIs of the same year have produced inconsistent results, these different results are unlikely to be attributed to variation in the years of HEI updates. For example, while our study reported a significant inverse association between HEI-2015 and incident T2D, a recent study reported no association [11]. A potential explanation for the heterogeneity in findings is differences in study population characteristics, as there was some heterogeneity in ethnicity and sex assigned at birth across studies.
Similar to our findings, previous evidence suggests that aMED is inversely associated with T2D incidence [4–6]. The observed inverse relationship between DASH and incident T2D in the present study is consistent with findings from the Health Professional’s Follow Up Study (HPFS) and Women’s Health Initiative (WHI) [4–5]. However, in the Insulin Resistance Atherosclerosis Study, DASH was inversely associated with T2D only among White participants and not among Black or Hispanic participants. In the InterAct Consortium conducted in Europe, higher DASH scores were not statistically significantly associated with risk of T2D [31–32]. Consistent with our findings, significant inverse associations between aHEI and T2D were observed in several studies, including the HPFS, Nurses’ Health Study, and WHI [4–5,8]. However, some studies have observed no association between aHEI and T2D risk [11, 32].
In line with our findings, previous studies have reported associations between SES indicators and T2D risk [12–15]. Clustering of adverse health behaviors that are risk factors for T2D, such as smoking, physical inactivity, and obesity, is more likely to be reported among those with low SES than those with high SES [33–35]. In addition, individuals with higher educational attainment and income tend to consume higher quality, nutrient-dense diets than those with lower income and educational attainment [36–37].
We found evidence of interaction between HEI-2015 and ADI on both additive and multiplicative scales. In stratified models, the inverse associations between dietary pattern scores and T2D were attenuated among those with lower SES and higher neighborhood deprivation. A previous study in a Dutch population corroborates these findings where associations between diet quality and incident T2D were weakest for individuals with the lowest educational attainment [16]. Similarly, a recent study found that the DASH dietary pattern was not associated with risk of heart failure among lowincome individuals but was inversely associated among higher-income participants [38]. Though neighborhood SES indicators have been associated with T2D risk, this study is the first to examine whether the association between healthy dietary indices and T2D risk varies by ADI [14–15]. ADI represents a combination of neighborhood factors, including income, employment, safety, access, and availability of resources that influence T2D risk. Due to the combination of risk factors associated with high neighborhood deprivation, it is possible that the benefits of adhering to healthy eating patterns do not outweigh the health costs of living in areas with high deprivation [16, 38].
Inverse associations between dietary indices and risk of T2D were fairly consistent across different racial and ethnic groups, except that the HEI-2015 was not associated with T2D among NHB. We found a significant inverse association between aMED and incident T2D risk across all racial and ethnic groups, but this association was most pronounced among women who were neither NHW nor NHB. In contrast, O’Connor et al. observed the inverse relationship between aMED and incident T2D was strongest among NHB women in the Atherosclerosis Risk in Communities study [6]. However, other studies have reported no association between healthy dietary indices (e.g., aMED, DASH, aHEI, and HEI-2010) and incident T2D among NHB women [7] or a combined race and ethnicity category of NHB and Hispanic women [31]. Future studies in diverse populations are needed to confirm these findings.
This study had several strengths. The prospective study design minimized the potential for reverse causality. Given the large sample size, we had the statistical power to detect differences in the association between dietary indices and incident T2D within strata of potential effect modifiers. Due to the comprehensive data collection methods in the Sister Study, we were able to adjust for multiple confounders. Additionally, the attrition rate in the Sister Study is low. Despite these strengths, dietary data were only collected at baseline, and we were unable to capture changes in dietary habits over time. Diabetes status was based on self-report, which left our study susceptible to misclassification bias. However, self-reported T2D has relatively high negative (> 90%) and positive (~ 80%) predictive values among women in the US [39]. In addition, evaluation of hemoglobin A1C levels in a subset of our population suggested that undetected T2D was limited [40].
In conclusion, higher diet quality, as assessed by four dietary indices was associated with reduced risk of T2D, and associations were strongest among women with higher income/lower neighborhood deprivation. Attenuated associations among participants with lower individual SES and higher neighborhood deprivation suggest that other risk factors play a larger role in T2D incidence than diet quality among individuals with low SES. Future studies are needed to confirm the potential socioeconomic disparities in the relationship between alignment with healthy eating patterns and T2D risk.
Supplementary Material
ACKNOWLEDGEMENTS
Funding:
BC is supported by grant number T32-GM081740 from NIH-NIGMS. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH. This work was supported, in part, by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences [Z01-ES044005]. This work was also partially supported by a grant from the Arkansas Breast Cancer Research Program.
Footnotes
Ethics Approval. The Sister Study is overseen by the National Institutes of Health Institutional Review Board.
Consent to Participate. All participants provided written informed consent.
Data and Resource Availability. The data sets generated during and/or analyzed during the current study are not publicly available due to privacy concerns. However, requests for data, including the data used in this analysis, may be made following procedures described on the Sister Study website (www.sisterstudy.niehs.nih.gov) under the tab “For Researchers.”
Competing Interests. The authors have no relevant financial or non-financial interests to disclose.
Declarations of Interest: The authors have no relevant financial or non-financial interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- [1].Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- [3].Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151 [DOI] [PubMed] [Google Scholar]
- [4].Cespedes EM, Hu FB, Tinker L, et al. Multiple Healthful Dietary Patterns and Type 2 Diabetes in the Women’s Health Initiative. Am J Epidemiol. 2016;183(7):622–633. doi: 10.1093/aje/kwv241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].de Koning L, Chiuve SE, Fung TT, Willett WC, Rimm EB, Hu FB. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care. 2011;34(5):1150–1156. doi: 10.2337/dc10-2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].O’Connor LE, Hu EA, Steffen LM, Selvin E, Rebholz CM. Adherence to a Mediterranean-style eating pattern and risk of diabetes in a U.S. prospective cohort study. Nutr Diabetes. 2020;10(1):8. doi: 10.1038/s41387-020-0113-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jacobs S, Boushey CJ, Franke AA, et al. A priori-defined diet quality indices, biomarkers and risk for type 2 diabetes in five ethnic groups: the Multiethnic Cohort. Br J Nutr. 2017;118(4):312–320. doi: 10.1017/S0007114517002033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. doi: 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schwingshackl L, Bogensberger B, Hoffmann G. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: An Updated Systematic Review and Meta-Analysis of Cohort Studies. J Acad Nutr Diet. 2018;118(1):74–100.e11. doi: 10.1016/j.jand.2017.08.024 [DOI] [PubMed] [Google Scholar]
- [10].Salas-Salvadó J, Bulló M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial [published correction appears in Diabetes Care. 2018 Oct;41(10):2259–2260]. Diabetes Care. 2011;34(1):14–19. doi: 10.2337/dc10-1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xu Z, Steffen LM, Selvin E, Rebholz CM. Diet quality, change in diet quality and risk of incident CVD and diabetes. Public Health Nutr. 2020;23(2):329–338. doi: 10.1017/S136898001900212X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. 2011;40(3):804–818. doi: 10.1093/ije/dyr029 [DOI] [PubMed] [Google Scholar]
- [13].Hwang J, Shon C. Relationship between socioeconomic status and type 2 diabetes: results from Korea National Health and Nutrition Examination Survey (KNHANES) 2010–2012. BMJ Open. 2014;4(8):e005710. doi: 10.1136/bmjopen2014-005710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bilal U, Hill-Briggs F, Sánchez-Perruca L, Del Cura-González I, Franco M. Association of neighbourhood socioeconomic status and diabetes burden using electronic health records in Madrid (Spain): the HeartHealthyHoods study. BMJ Open. 2018;8(9):e021143. doi: 10.1136/bmjopen-2017-021143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Consolazio D, Koster A, Sarti S, et al. Neighbourhood property value and type 2 diabetes mellitus in the Maastricht study: A multilevel study. PLoS One. 2020;15(6):e0234324. doi: 10.1371/journal.pone.0234324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vinke PC, Navis G, Kromhout D, Corpeleijn E. Socio-economic disparities in the association of diet quality and type 2 diabetes incidence in the Dutch Lifelines cohort. EClinicalMedicine. 2020;19:100252. doi: 10.1016/j.eclinm.2019.100252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sandler DP, Hodgson ME, Deming-Halverson SL, et al. The Sister Study Cohort: Baseline Methods and Participant Characteristics. Environ Health Perspect. 2017;125(12):127003. doi: 10.1289/EHP1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–469. doi: 10.1093/oxfordjournals.aje.a114416 [DOI] [PubMed] [Google Scholar]
- [19].Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006;9(1):84–93. doi: 10.1079/phn2005763 [DOI] [PubMed] [Google Scholar]
- [20].Bowman SA, Clemens JC, Friday JE, Thoerig RC,Moshfegh AJ. Food patterns equivalents database 2011–12: Methodology and user guide [Online]. Food Surveys Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture, Beltsville, Maryland. 2014. Available at http://www.ars.usda.gov/nea/bhnrc/fsrg. Accessed 12 February 2022. [Google Scholar]
- [21].Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015 [published correction appears in J Acad Nutr Diet. 2019 Aug 20;:]. J Acad Nutr Diet. 2018;118(9):1591–1602. doi: 10.1016/j.jand.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women [published correction appears in Circulation. 2009;119(12):e379]. Circulation. 2009;119(8):1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–2608. doi: 10.1056/NEJMoa025039 [DOI] [PubMed] [Google Scholar]
- [24].Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women [published correction appears in Arch Intern Med. 2008 Jun 23;168(12):1276]. Arch Intern Med. 2008;168(7):713–720. doi: 10.1001/archinte.168.7.713 [DOI] [PubMed] [Google Scholar]
- [25].Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93(7):1137–1143. doi: 10.2105/ajph.93.7.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kind AJH, Buckingham WR. Making Neighborhood-Disadvantage Metrics Accessible - The Neighborhood Atlas. N Engl J Med. 2018;378(26):2456–2458. doi: 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sharma M, Petersen I, Nazareth I, Coton SJ. An algorithm for identification and classification of individuals with type 1 and type 2 diabetes mellitus in a large primary care database. Clin Epidemiol. 2016;8:373–380. doi: 10.2147/CLEP.S113415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Garger YB, Joshi PM, Pareek AS, et al. Secondary causes of diabetes mellitus. In: Poretsky L, ed. Principles of Diabetes Mellitus. Boston, MA: Springer US; 2010:245–258 [Google Scholar]
- [29].VanderWeele TJ and Knol MJ. A tutorial on interaction. Epidemiologic Methods. 2014;3(1):33–72. doi: 10.1515/em-2013-0005 [DOI] [Google Scholar]
- [30].Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–456. doi: 10.1097/00001648199209000-00012 [DOI] [PubMed] [Google Scholar]
- [31].Liese AD, Nichols M, Sun X, D’Agostino RB Jr, Haffner SM. Adherence to the DASH Diet is inversely associated with incidence of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care. 2009;32(8):1434–1436. doi: 10.2337/dc09-0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Consortium InterAct. Adherence to predefined dietary patterns and incident type 2 diabetes in European populations: EPIC-InterAct Study. Diabetologia. 2014;57(2):321–333. doi: 10.1007/s00125-013-3092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hiscock R, Bauld L, Amos A, Fidler JA, Munafò M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci. 2012;1248:107–123. doi: 10.1111/j.1749-6632.2011.06202.x [DOI] [PubMed] [Google Scholar]
- [34].Huikari S, Junttila H, Ala-Mursula L, et al. Leisure-time physical activity is associated with socio-economic status beyond income - Cross-sectional survey of the Northern Finland Birth Cohort 1966 study. Econ Hum Biol. 2021;41:100969. doi: 10.1016/j.ehb.2020.100969 [DOI] [PubMed] [Google Scholar]
- [35].Mohammed SH, Habtewold TD, Birhanu MM, et al. Neighbourhood socioeconomic status and overweight/obesity: a systematic review and meta-analysis of epidemiological studies. BMJ Open. 2019;9(11):e028238. doi: 10.1136/bmjopen2018-028238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mullie P, Clarys P, Hulens M, Vansant G. Dietary patterns and socioeconomic position. Eur J Clin Nutr. 2010;64(3):231–238. doi: 10.1038/ejcn.2009.145 [DOI] [PubMed] [Google Scholar]
- [37].Darmon N, Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. 2015;73(10):643–660. doi: 10.1093/nutrit/nuv027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chang RS, Xu M, Brown SH, et al. Relation of the Dietary Approaches to Stop Hypertension Dietary Pattern to Heart Failure Risk and Socioeconomic Status (from the Southern Community Cohort Study). Am J Cardiol. 2022;169:71–77. doi: 10.1016/j.amjcard.2021.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jackson JM, DeFor TA, Crain AL, et al. Validity of diabetes self-reports in the Women’s Health Initiative. Menopause. 2014;21(8):861–868. doi: 10.1097/GME.0000000000000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Park YM, Bookwalter DB, O’Brien KM, Jackson CL, Weinberg CR, Sandler DP. A prospective study of type 2 diabetes, metformin use, and risk of breast cancer. Ann Oncol. 2021;32(3):351–359. doi: 10.1016/j.annonc.2020.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.