Abstract
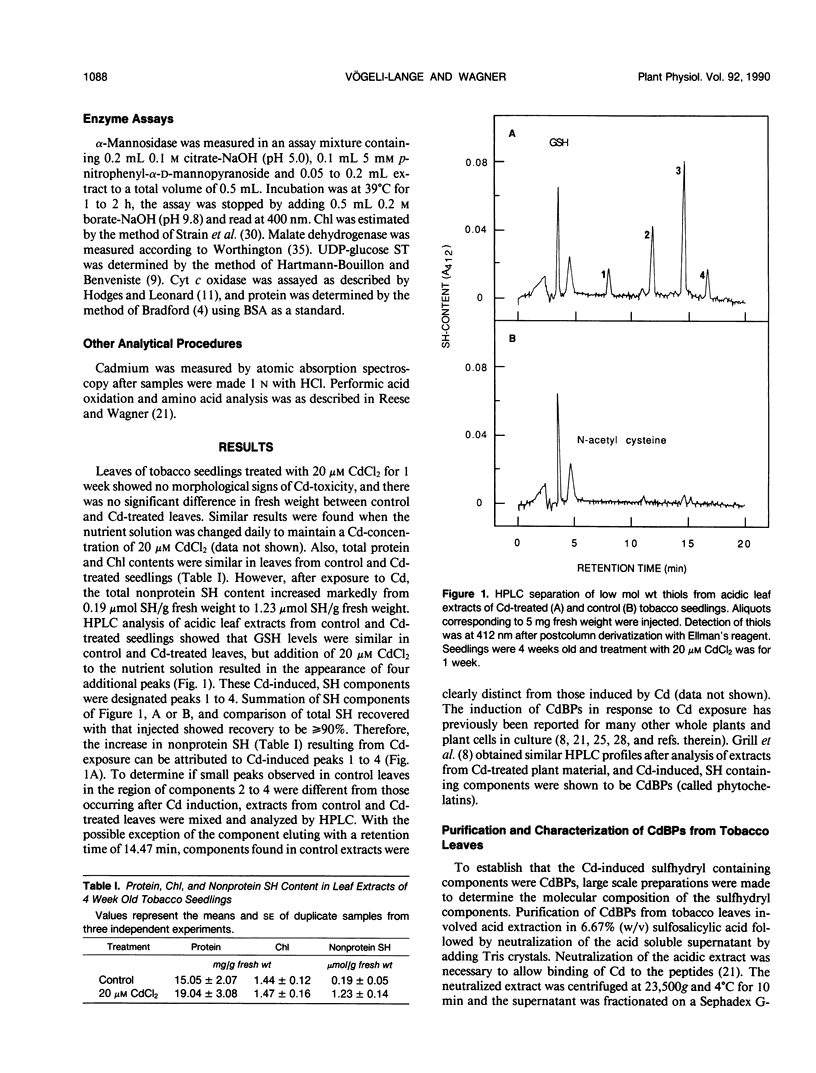
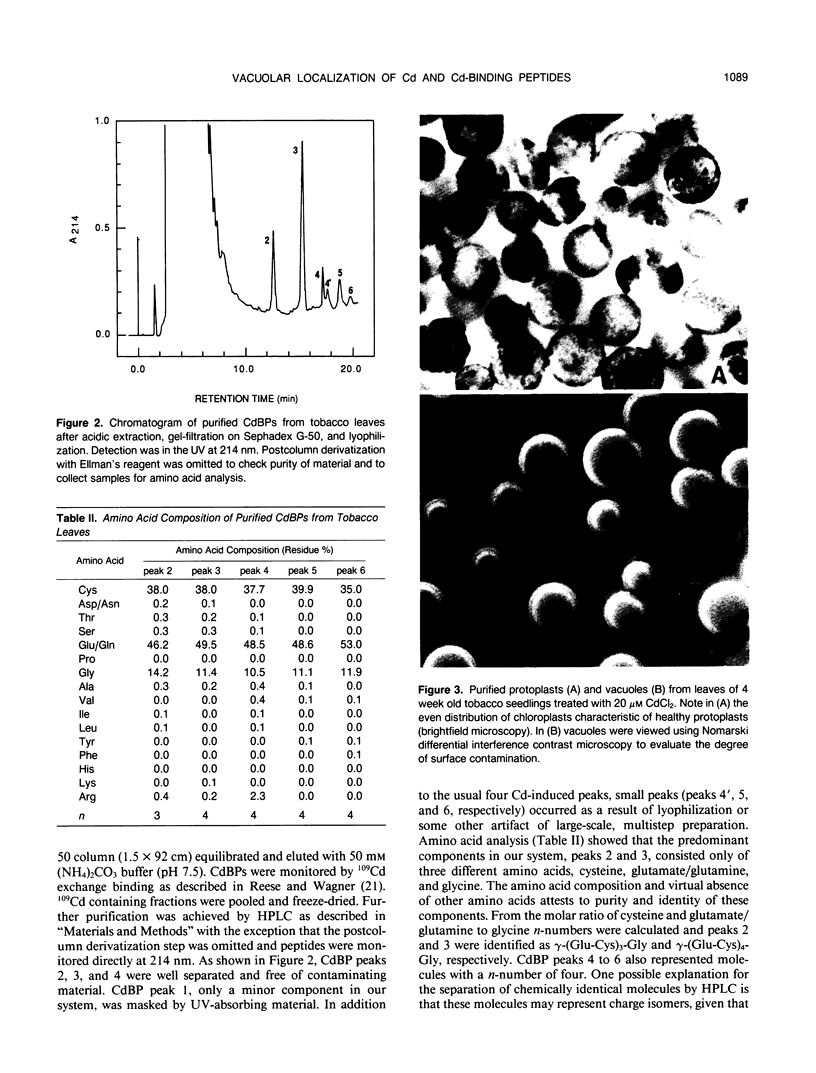
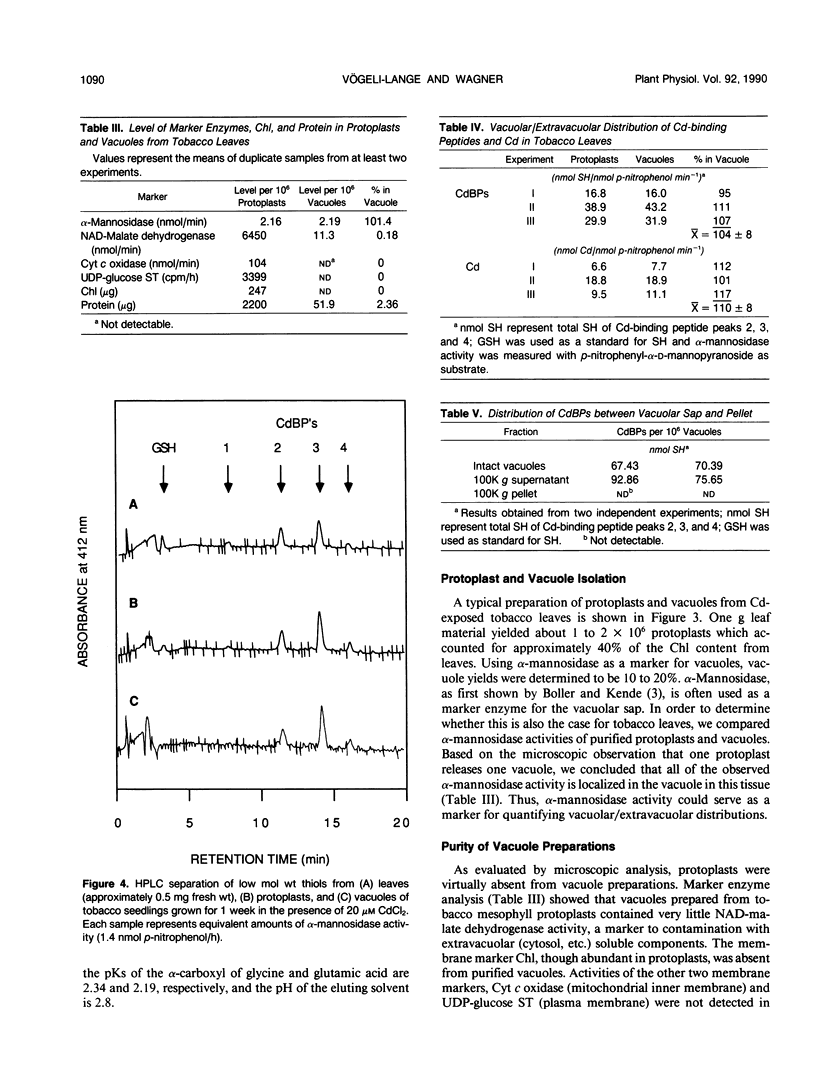
The synthesis of Cd-binding peptides (CdBPs) was induced upon addition of 20 micromolar CdCl2 (nonphytotoxic level) to the nutrient solution of hydroponically grown tobacco seedlings (Nicotiana rustica var Pavonii). Amino acid analysis showed that the main components were γ-(Glu-Cys)3-Gly and γ-(Glu-Cys)4-Gly. Seedlings exposed to the metal for 1 week contained similar glutathione levels as found in the controls (about 0.18 micromole per gram fresh weight). If, as has been proposed, CdBPs are involved in Cd-detoxification by chelation, both metal and ligand must be localized in the same cellular compartment. To directly determine the localization of Cd and CdBPs, protoplasts and vacuoles were isolated from leaves of Cd-exposed seedlings. Purified vacuoles contained virtually all of the CdBPs and Cd found in protoplasts (104% ± 8 and 110% ± 8, respectively). CdBPs were associated with the vacuolar sap and not with the tonoplast membrane. Glutathione was observed in leaves and protoplasts but not in vacuoles. The probability that CdBPs are synthesized extravacuolarly and our finding that they and Cd are predominantly located in the vacuole suggest that these molecules might be involved in transport of Cd to the vacuole. Our results also suggest that a simple cytoplasmic chelator role for CdBPs in Cd tolerance cannot be assumed.
Full text
PDF

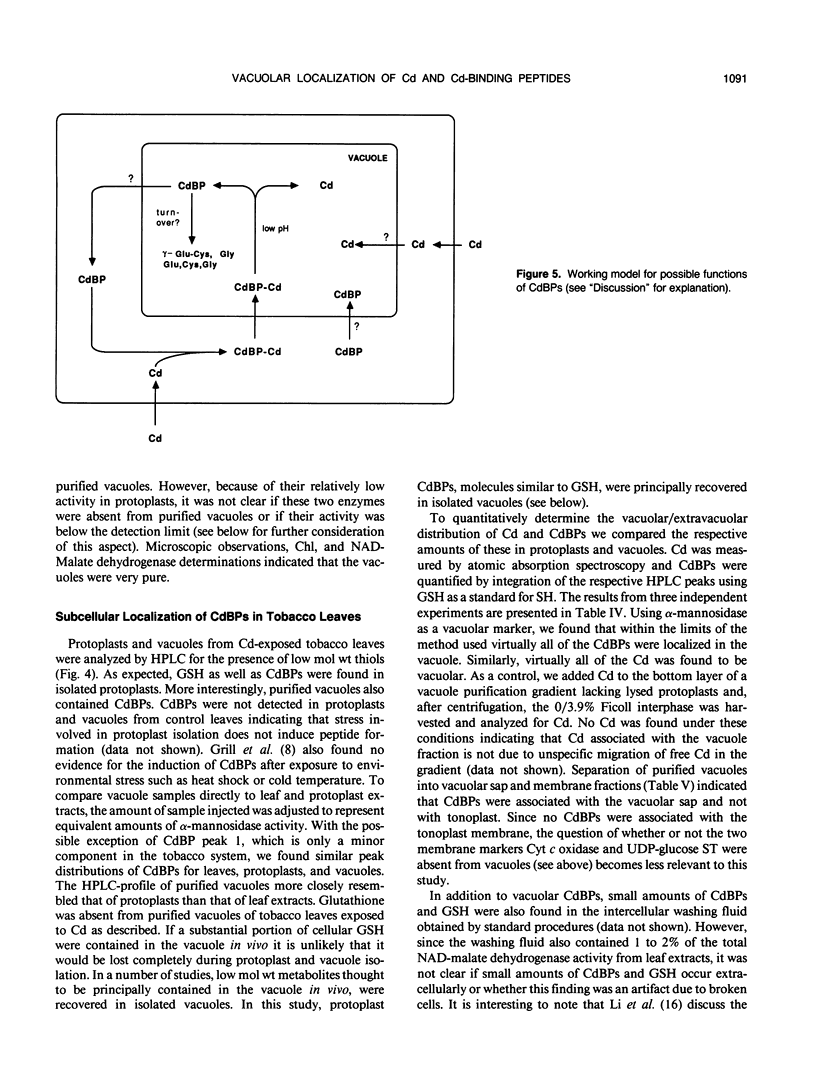


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumwald E., Poole R. J. Na/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris. Plant Physiol. 1985 May;78(1):163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cataldo D. A., Garland T. R., Wildung R. E. Cadmium distribution and chemical fate in soybean plants. Plant Physiol. 1981 Oct;68(4):835–839. doi: 10.1104/pp.68.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E., Jackson P. J., Lujan L. D., Robinson N. J. Poly(gamma-glutamylcysteinyl)glycine Synthesis in Datura innoxia and Binding with Cadmium : Role in Cadmium Tolerance. Plant Physiol. 1989 Feb;89(2):700–706. doi: 10.1104/pp.89.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E., Winnacker E. L., Zenk M. H. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci U S A. 1987 Jan;84(2):439–443. doi: 10.1073/pnas.84.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Krotz R. M., Evangelou B. P., Wagner G. J. Relationships between Cadmium, Zinc, Cd-Peptide, and Organic Acid in Tobacco Suspension Cells. Plant Physiol. 1989 Oct;91(2):780–787. doi: 10.1104/pp.91.2.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. C., McClure J. W., Hagerman A. E. Soluble and Bound Apoplastic Activity for Peroxidase, beta-d-Glucosidase, Malate Dehydrogenase, and Nonspecific Arylesterase, in Barley (Hordeum vulgare L.) and Oat (Avena sativa L.) Primary Leaves. Plant Physiol. 1989 May;90(1):185–190. doi: 10.1104/pp.90.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoh T., Watanabe J., Takahashi E. Sodium, Potassium, Chloride, and Betaine Concentrations in Isolated Vacuoles from Salt-Grown Atriplex gmelini Leaves. Plant Physiol. 1987 May;84(1):173–177. doi: 10.1104/pp.84.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R. S., Gray R. H., Chin B., Bernstein I. A. Molecular mechanisms of accommodation in Escherichia coli to toxic levels of Cd2+. J Bacteriol. 1975 Mar;121(3):1180–1188. doi: 10.1128/jb.121.3.1180-1188.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R. N., Wagner G. J. Effects of buthionine sulfoximine on cd-binding Peptide levels in suspension-cultured tobacco cells treated with cd, zn, or cu. Plant Physiol. 1987 Jul;84(3):574–577. doi: 10.1104/pp.84.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R. N., Wagner G. J. Properties of tobacco (Nicotiana tabacum) cadmium-binding peptide(s). Unique non-metallothionein cadmium ligands. Biochem J. 1987 Feb 1;241(3):641–647. doi: 10.1042/bj2410641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R. N., Winge D. R. Sulfide stabilization of the cadmium-gamma-glutamyl peptide complex of Schizosaccharomyces pombe. J Biol Chem. 1988 Sep 15;263(26):12832–12835. [PubMed] [Google Scholar]
- Scheller H. V., Huang B., Hatch E., Goldsbrough P. B. Phytochelatin synthesis and glutathione levels in response to heavy metals in tomato cells. Plant Physiol. 1987 Dec;85(4):1031–1035. doi: 10.1104/pp.85.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Calcium transport into the vacuole of oat roots. Characterization of H+/Ca2+ exchange activity. J Biol Chem. 1986 Sep 15;261(26):12172–12178. [PubMed] [Google Scholar]
- Sela M., Tel-Or E., Fritz E., Huttermann A. Localization and toxic effects of cadmium, copper, and uranium in azolla. Plant Physiol. 1988 Sep;88(1):30–36. doi: 10.1104/pp.88.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens J. C., Hunt D. F., Williams B. G. Accumulation of non-protein metal-binding polypeptides (gamma-glutamyl-cysteinyl)n-glycine in selected cadmium-resistant tomato cells. J Biol Chem. 1986 Oct 25;261(30):13879–13882. [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Energy-dependent efflux of cadmium coded by a plasmid resistance determinant in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):313–319. doi: 10.1128/jb.147.2.313-319.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel H. J., Jäger H. J. Subcellular distribution and chemical form of cadmium in bean plants. Plant Physiol. 1980 Mar;65(3):480–482. doi: 10.1104/pp.65.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]