Abstract
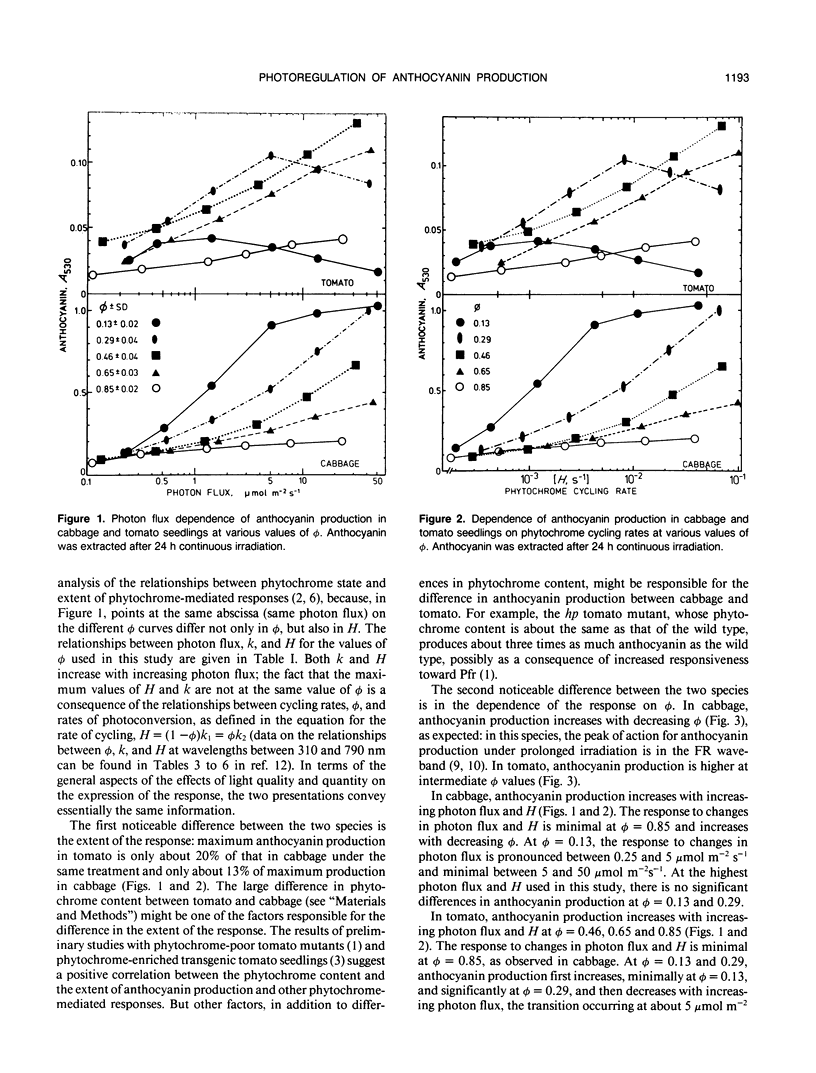
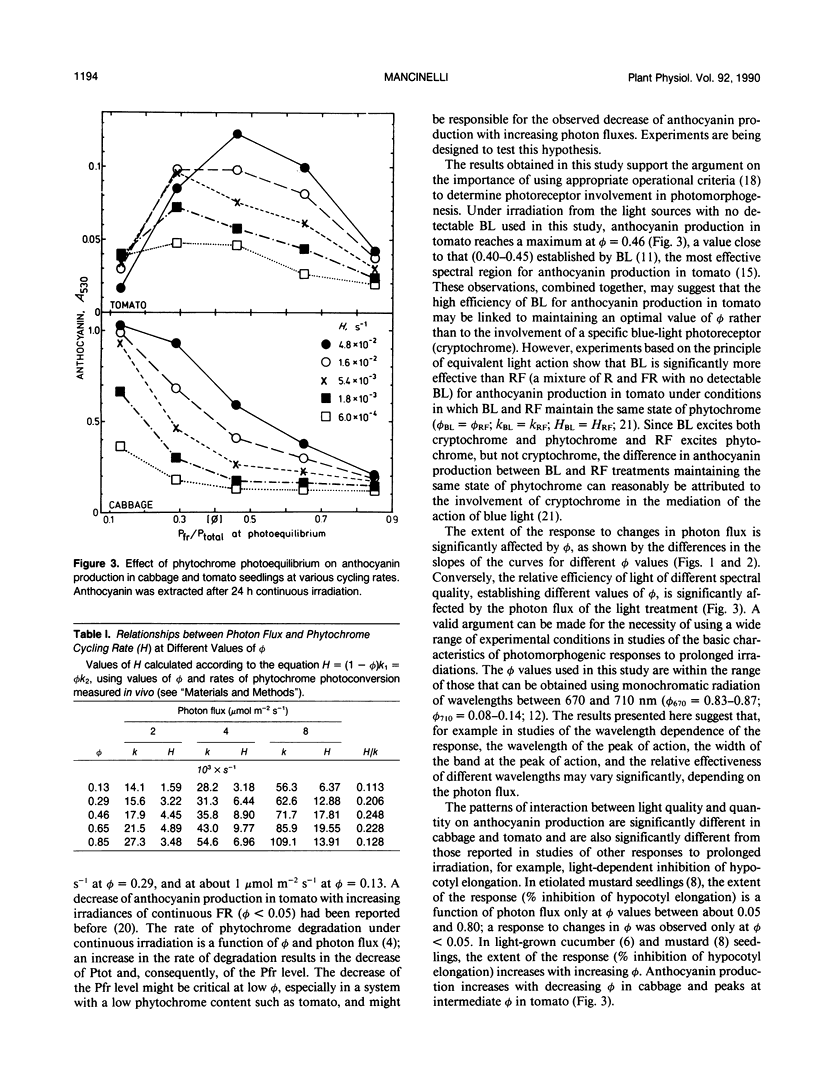
The interaction between phytochrome photoequilibrium (φ) and photon flux in the photoregulation of anthocyanin production under prolonged irradiation was studied in seedlings of Brassica oleracea L. and Lycopersicon esculentum Mill. In cabbage, anthocyanin production increases with decreasing φ, reaching a maximum at the lowest value (φ = 0.13) used in this study; in tomato, the extent of the response is higher at intermediate values, reaching a maximum at φ = 0.46. In cabbage, the response increases with increasing photon flux at all φ values; however, the response to changes in photon flux is minimal at φ = 0.85, and, at φ = 0.13, minimal at photon fluxes higher than 5 micromolar per square meter per second. In tomato, the response increases with increasing photon flux at φ = 0.46, 0.65, and 0.85, the response to changes in photon fluxes being minimal at φ = 0.85; at φ = 0.13 and 0.29 the response first increases (significantly at φ = 0.29 and minimally at φ = 0.13) and then decreases with increasing photon fluxes, the transition occurring at about 1 micromolar per square meter per second at φ = 0.13, and at 5 micromolar per square meter per second at φ = 0.29. The patterns of light quality-quantity interaction in the photoregulation of anthocyanin production are significantly different in cabbage and tomato and are also significantly different than those observed for other photomorphogenic responses to prolonged irradiations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylan M. T., Quail P. H. Oat Phytochrome Is Biologically Active in Transgenic Tomatoes. Plant Cell. 1989 Aug;1(8):765–773. doi: 10.1105/tpc.1.8.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaba V., Black M. Photocontrol of Hypocotyl Elongation in Light-Grown Cucumis sativus L. : Responses to Phytochrome Photostationary State and Fluence Rate. Plant Physiol. 1985 Dec;79(4):1011–1014. doi: 10.1104/pp.79.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli A. L. Phytochrome Photoconversion in Vivo: Comparison between Measured and Predicted Rates. Plant Physiol. 1988 Mar;86(3):749–753. doi: 10.1104/pp.86.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli A. L., Walsh L. Photocontrol of Anthocyanin Synthesis: VII. Factors Affecting the Spectral Sensitivity of Anthocyanin Synthesis in Young Seedlings. Plant Physiol. 1979 May;63(5):841–846. doi: 10.1104/pp.63.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli A. L., Yang C. P., Lindquist P., Anderson O. R., Rabino I. Photocontrol of Anthocyanin Synthesis: III. The Action of Streptomycin on the Synthesis of Chlorophyll and Anthocyanin. Plant Physiol. 1975 Feb;55(2):251–257. doi: 10.1104/pp.55.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabino I., Mancinelli A. L., Kuzmanoff K. M. Photocontrol of Anthocyanin Synthesis: VI. Spectral Sensitivity, Irradiance Dependence, and Reciprocity Relationships. Plant Physiol. 1977 Apr;59(4):569–573. doi: 10.1104/pp.59.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponga F., Deitzer G. F., Mancinelli A. L. Cryptochrome, Phytochrome, and the Photoregulation of Anthocyanin Production under Blue Light. Plant Physiol. 1986 Dec;82(4):952–955. doi: 10.1104/pp.82.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]


