Abstract
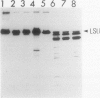
The lysate from intact chloroplasts mechanically isolated from primary leaves of 9 day old seedlings of wheat (Triticum aestivum L. var Aoba) was incubated in the pH range of 5.5 to 8.5 at 37°C for 5 hours. Proteolytic activity against ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco, EC 4.1.1.39) was estimated by disappearance of the large subunit of Rubisco or the appearance of its degradation products. Although the activity in lysates was weak, the products were detected by applying Western blotting. The degradation products were similar to those obtained when Rubisco was incubated with the lysate of vacuoles isolated from like leaves. Although some of the products were similar to those from vacuole lysates, many were clearly different after incubation of Rubisco with trypsin, V-8 protease, or reactive oxygen (hydroxy radical). Lysates of chloroplasts, pretreated with thermolysin at 4°C for 30 minutes, had no proteolytic activity against Rubisco after incubation at 37°C for 5 hours. These results show that the proteolytic activity against Rubisco found in lysates of our mechanically isolated chloroplasts was mostly due to the contamination of vacuolar proteases adhering to the outer envelope of the chloroplasts during their isolation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmair A., Finley D., Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986 Oct 10;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Andrews J., Keegstra K. Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol. 1984 Jul;75(3):675–678. doi: 10.1104/pp.75.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. J., Goldberg A. L. Oxygen radicals stimulate intracellular proteolysis and lipid peroxidation by independent mechanisms in erythrocytes. J Biol Chem. 1987 Jun 15;262(17):8220–8226. [PubMed] [Google Scholar]
- Davies K. J., Goldberg A. L. Proteins damaged by oxygen radicals are rapidly degraded in extracts of red blood cells. J Biol Chem. 1987 Jun 15;262(17):8227–8234. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makino A., Mae T., Ohira K. Photosynthesis and Ribulose 1,5-Bisphosphate Carboxylase in Rice Leaves: Changes in Photosynthesis and Enzymes Involved in Carbon Assimilation from Leaf Development through Senescence. Plant Physiol. 1983 Dec;73(4):1002–1007. doi: 10.1104/pp.73.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivett A. J. Preferential degradation of the oxidatively modified form of glutamine synthetase by intracellular mammalian proteases. J Biol Chem. 1985 Jan 10;260(1):300–305. [PubMed] [Google Scholar]
- Snyder M., Elledge S., Sweetser D., Young R. A., Davis R. W. Lambda gt 11: gene isolation with antibody probes and other applications. Methods Enzymol. 1987;154:107–128. doi: 10.1016/0076-6879(87)54073-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardley T. M., Bhalla P. L., Dalling M. J. Changes in the Number and Composition of Chloroplasts during Senescence of Mesophyll Cells of Attached and Detached Primary Leaves of Wheat (Triticum aestivum L.). Plant Physiol. 1984 Jun;75(2):421–424. doi: 10.1104/pp.75.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A., Lin W., Hebert R. R. Vacuolar localization of proteases and degradation of chloroplasts in mesophyll protoplasts from senescing primary wheat leaves. Plant Physiol. 1982 Jan;69(1):98–102. doi: 10.1104/pp.69.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]