Abstract
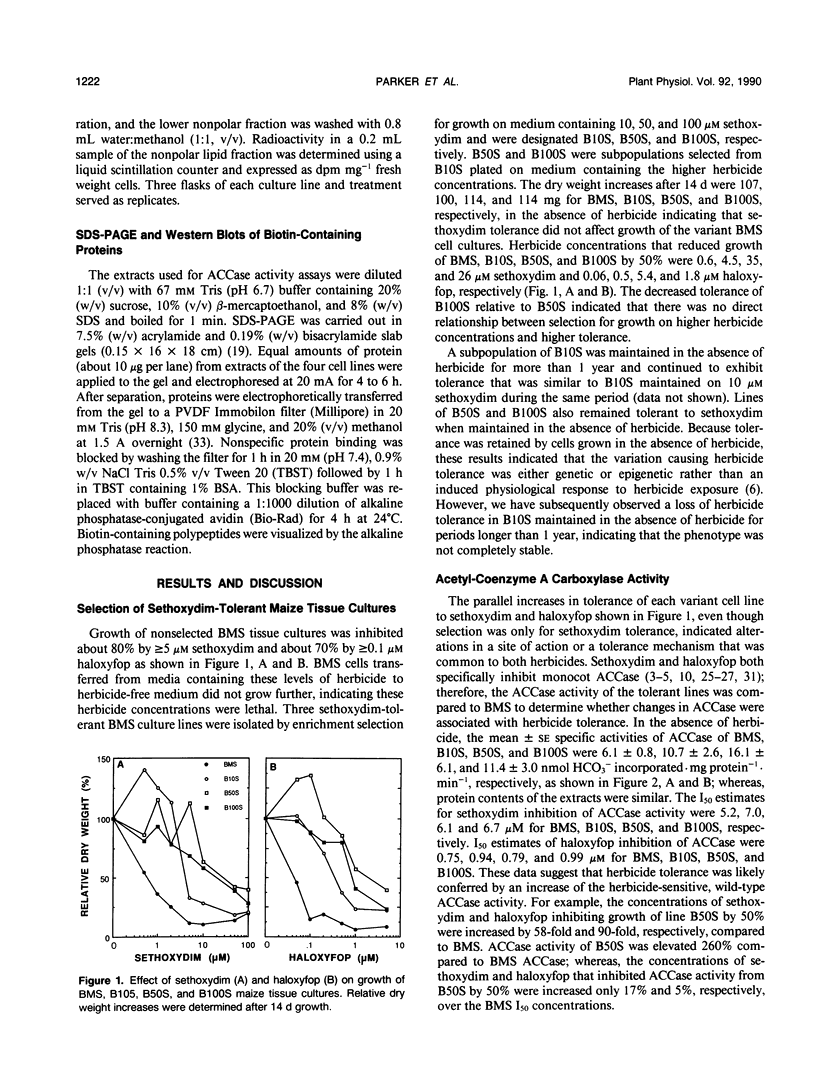
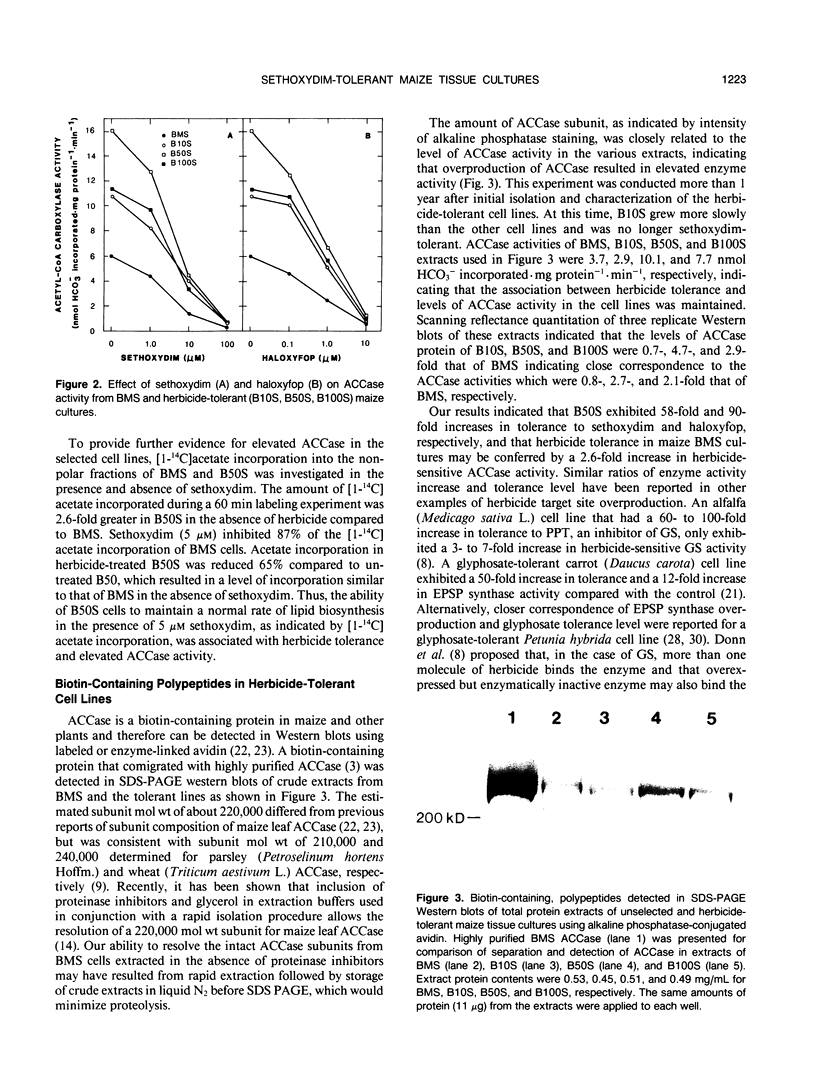
`Black Mexican Sweet' (BMS) maize (Zea mays L.) tissue cultures were selected for tolerance to sethoxydim. Sethoxydim, a cyclohexanedione, and haloxyfop, an aryloxyphenoxypropionate, exert herbicidal activity on most monocots including maize by inhibiting acetyl-coenzyme A carboxylase (ACCase). Selected line B10S grew on medium containing 10 micromolar sethoxydim. Lines B50S and B100S were subsequent selections from B10S that grew on medium containing 50 and 100 micromolar sethoxydim, respectively. Growth rates of BMS, B10S, B50S, and B100S were similar in the absence of herbicide. Herbicide concentrations reducing growth by 50% were 0.6, 4.5, 35, and 26 micromolar sethoxydim and 0.06, 0.5, 5.4, and 1.8 micromolar haloxyfop for BMS, B10S, B50S, and B100S, respectively. Sethoxydim and haloxyfop concentrations that inhibited ACCase by 50% were similar for BMS, B10S, B50S, and B100S. However, ACCase activities were 6.01, 10.7, 16.1, and 11.4 nmol HCO3− incorporated per milligram of protein per minute in extracts of BMS, B10S, B50S, and B100S, respectively, suggesting that increased wild-type ACCase activity conferred herbicide tolerance. Incorporation of [14C]acetate into the nonpolar lipid fraction was higher for B50S than for BMS in the absence of sethoxydim providing further evidence for an increase in ACCase activity in the selected line. In the presence of 5 micromolar sethoxydim, [14C]acetate incorporation by B50S was similar to that for untreated BMS. The levels of a biotin-containing polypeptide (about 220,000 molecular weight), presumably the ACCase subunit, were increased in the tissue cultures that exhibited elevated ACCase activity indicating overproduction of the ACCase enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton J. D., Gronwald J. W., Somers D. A., Connelly J. A., Gengenbach B. G., Wyse D. L. Inhibition of plant acetyl-coenzyme A carboxylase by the herbicides sethoxydim and haloxyfop. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1039–1044. doi: 10.1016/s0006-291x(87)80236-x. [DOI] [PubMed] [Google Scholar]
- Donn G., Tischer E., Smith J. A., Goodman H. M. Herbicide-resistant alfalfa cells: an example of gene amplification in plants. J Mol Appl Genet. 1984;2(6):621–635. [PubMed] [Google Scholar]
- Egin-Bühler B., Loyal R., Ebel J. Comparison of acetyl-CoA carboxylases from parsley cell cultures and wheat germ. Arch Biochem Biophys. 1980 Aug;203(1):90–100. doi: 10.1016/0003-9861(80)90156-3. [DOI] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellyer A., Bambridge H. E., Slabas A. R. Plant acetyl-CoA carboxylase. Biochem Soc Trans. 1986 Jun;14(3):565–568. doi: 10.1042/bst0140565. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nafziger E. D., Widholm J. M., Steinrücken H. C., Killmer J. L. Selection and characterization of a carrot cell line tolerant to glyphosate. Plant Physiol. 1984 Nov;76(3):571–574. doi: 10.1104/pp.76.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolau B. J., Hawke J. C. Purification and characterization of maize leaf acetyl-coenzyme A carboxylase. Arch Biochem Biophys. 1984 Jan;228(1):86–96. doi: 10.1016/0003-9861(84)90049-3. [DOI] [PubMed] [Google Scholar]
- Nikolau B. J., Wurtele E. S., Stumpf P. K. Tissue distribution of acetyl-coenzyme a carboxylase in leaves. Plant Physiol. 1984 Aug;75(4):895–901. doi: 10.1104/pp.75.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendina A. R., Felts J. M., Beaudoin J. D., Craig-Kennard A. C., Look L. L., Paraskos S. L., Hagenah J. A. Kinetic characterization, stereoselectivity, and species selectivity of the inhibition of plant acetyl-CoA carboxylase by the aryloxyphenoxypropionic acid grass herbicides. Arch Biochem Biophys. 1988 Aug 15;265(1):219–225. doi: 10.1016/0003-9861(88)90387-6. [DOI] [PubMed] [Google Scholar]
- Rendina A. R., Felts J. M. Cyclohexanedione Herbicides Are Selective and Potent Inhibitors of Acetyl-CoA Carboxylase from Grasses. Plant Physiol. 1988 Apr;86(4):983–986. doi: 10.1104/pp.86.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenn J. D., Schriek U. PAPS-reductase from Escherichia coli: characterization of the enzyme as probe for thioredoxins. Z Naturforsch C. 1987 Jan-Feb;42(1-2):93–102. doi: 10.1515/znc-1987-1-216. [DOI] [PubMed] [Google Scholar]
- Secor J., Cséke C. Inhibition of Acetyl-CoA Carboxylase Activity by Haloxyfop and Tralkoxydim. Plant Physiol. 1988 Jan;86(1):10–12. doi: 10.1104/pp.86.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. M., Horsch R. B., Klee H. J., Kishore G. M., Winter J. A., Tumer N. E., Hironaka C. M., Sanders P. R., Gasser C. S., Aykent S., Siegel N. R., Rogers S. G., Fraley R. T. Engineering herbicide tolerance in transgenic plants. Science. 1986 Jul 25;233(4762):478–481. doi: 10.1126/science.233.4762.478. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Steinrücken H. C., Schulz A., Amrhein N., Porter C. A., Fraley R. T. Overproduction of 5-enolpyruvylshikimate-3-phosphate synthase in a glyphosate-tolerant Petunia hybrida cell line. Arch Biochem Biophys. 1986 Jan;244(1):169–178. doi: 10.1016/0003-9861(86)90106-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]