Abstract
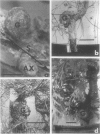
Two-to 4-month-old seedlings of nine pine species (Pinus eldarica Medw., Pinus elliottii Engelm., Pinus jeffreyi Grev. & Balf., Pinus lambertiana Dougl., Pinus ponderosa Laws., Pinus radiata D. Don, Pinus sylvestris L., Pinus taeda L., Pinus virginiana Mill), Douglas fir (Pseudotsuaa menziesii (Mirb.) Franco) and incense cedar (Libocedrus decurrens Torr.) were inoculated with five strains of Agrobacterium tumefaciens. Transformation occurred in all conifer species tested as determined by gall formation and opine production. The frequency of gall formation varied by host species, by bacterial strain, and was related to the age of the stem when inoculated. Galls were visible 8 to 12 weeks after inoculation and were small (often less than 2.5 millimeters in diameter). Fewer than half (230 of 502) of the galls originally formed on the trees were present after 1 year, and 26 of these grew to diameters greater than 2 centimeters. The majority of these larger galls (18 of 26) were found in P. radiata. Bacterial strain-specific opines were found in 67 of the 81 gall tissues sampled.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi D. E., Morris R. O., Hinz R., Mischke B. S., Kosuge T., Garfinkel D. J., Gordon M. P., Nester E. W. Cytokinin/auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc Natl Acad Sci U S A. 1983 Jan;80(2):407–411. doi: 10.1073/pnas.80.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. C., Kuleck G. A., Binns A. N. The Initiation of Auxin Autonomy in Tissue from Tobacco Plants Carrying the Auxin Biosynthesizing Genes from the T-DNA of Agrobacterium tumefaciens. Plant Physiol. 1986 Jan;80(1):145–151. doi: 10.1104/pp.80.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou P., Platt S. G., Ackerman M. C. Opine synthesis in wild-type plant tissue. Plant Physiol. 1986 Sep;82(1):218–221. doi: 10.1104/pp.82.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon P., Chilton M. D., Petit A., Tempé J. Agropine in "null-type" crown gall tumors: Evidence for generality of the opine concept. Proc Natl Acad Sci U S A. 1980 May;77(5):2693–2697. doi: 10.1073/pnas.77.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn A. G., Clarke L. E., Blundy K. S., White J. Nopaline Ti-plasmid, pTiT37, T-DNA insertions into a flax genome. J Mol Appl Genet. 1983;2(2):211–224. [PubMed] [Google Scholar]
- Kao J. C., Perry K. L., Kado C. I. Indoleacetic acid complementation and its relation to host range specifying genes on the Ti plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1982;188(3):425–432. doi: 10.1007/BF00330044. [DOI] [PubMed] [Google Scholar]
- Owens L. D., Cress D. E. Genotypic variability of soybean response to agrobacterium strains harboring the ti or ri plasmids. Plant Physiol. 1985 Jan;77(1):87–94. doi: 10.1104/pp.77.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarrelli J., Watters M. T., Diba L. H. Response of various cucurbits to infection by plasmid-harboring strains of agrobacterium. Plant Physiol. 1986 Oct;82(2):622–624. doi: 10.1104/pp.82.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]