Abstract
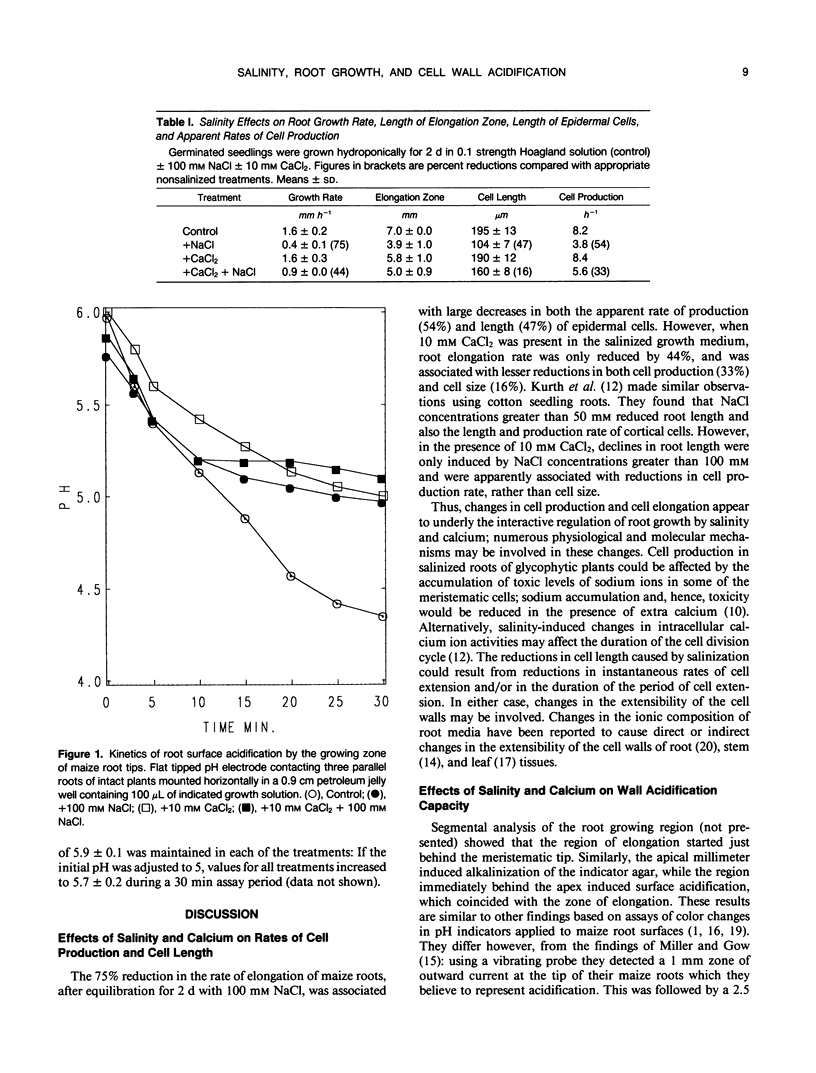
The reduction in growth of maize (Zea mays L.) seedling primary roots induced by salinization of the nutrient medium with 100 millimolar NaCl was accompanied by reductions in the length of the root tip elongation zone, the length of fully elongated epidermal cells, and the apparent rate of cell production: Each was partially restored when calcium levels in the salinized growth medium were increased from 0.5 to 10.0 millimolar. We investigated the possibility that the inhibition of elongation growth by salinity might be associated with an inhibition of cell wall acidification, such as that which occurs when root growth is inhibited by IAA. A qualitative assay of root surface acidification, using bromocresol purple pH indicator in agar, showed that salinized roots, with and without extra calcium, produced a zone of surface acidification which was similar to that produced by control roots. The zone of acidification began 1 to 2 millimeters behind the tip and coincided with the zone of cell elongation. The remainder of the root alkalinized its surface. Kinetics of surface acidification were assayed quantitatively by placing a flat tipped pH electrode in contact with the elongation zone. The pH at the epidermal surfaces of roots grown either with 100 millimolar NaCl (growth inhibitory), or with 10 millimolar calcium ± NaCl (little growth inhibition), declined from 6.0 to 5.1 over 30 minutes. We conclude that NaCl did not inhibit growth by reducing the capacity of epidermal cells to acidify their walls.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkman T., Leopold A. C. An electric current associated with gravity sensing in maize roots. Plant Physiol. 1987;84:841–846. doi: 10.1104/pp.84.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman J. M. Mechanisms of salinity tolerance in plants. Plant Physiol. 1988 Jul;87(3):547–550. doi: 10.1104/pp.87.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E., Cosgrove D., Tepfer M. Long-term acid-induced wall extension in an in-vitro system. Planta. 1987;170:379–385. [PubMed] [Google Scholar]
- Cleland R. E. Kinetics of Hormone-induced H Excretion. Plant Physiol. 1976 Aug;58(2):210–213. doi: 10.1104/pp.58.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer G. R., Läuchli A., Polito V. S. Displacement of ca by na from the plasmalemma of root cells : a primary response to salt stress? Plant Physiol. 1985 Sep;79(1):207–211. doi: 10.1104/pp.79.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstein K. H., Evans M. L. The influence of calcium and pH on growth in primary roots of Zea mays. Physiol Plant. 1988;72:466–470. doi: 10.1111/j.1399-3054.1988.tb09152.x. [DOI] [PubMed] [Google Scholar]
- Hassidim M., Braun Y., Lerner H. R., Reinhold L. Studies on H-Translocating ATPases in Plants of Varying Resistance to Salinity : II. K Strongly Promotes Development of Membrane Potential in Vesicles from Cotton Roots. Plant Physiol. 1986 Aug;81(4):1057–1061. doi: 10.1104/pp.81.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby B., Hanson J. B. Controls on na influx in corn roots. Plant Physiol. 1985 Apr;77(4):930–934. doi: 10.1104/pp.77.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham M. B., Gardner W. R., Gerloff G. C. Leaf water potential of differentially salinized plants. Plant Physiol. 1969 Oct;44(10):1378–1382. doi: 10.1104/pp.44.10.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth E., Cramer G. R., Läuchli A., Epstein E. Effects of NaCl and CaCl(2) on Cell Enlargement and Cell Production in Cotton Roots. Plant Physiol. 1986 Dec;82(4):1102–1106. doi: 10.1104/pp.82.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahaye P. A., Epstein E. Salt toleration by plants: enhancement with calcium. Science. 1969 Oct 17;166(3903):395–396. doi: 10.1126/science.166.3903.395. [DOI] [PubMed] [Google Scholar]
- Miller A. L., Gow N. A. Correlation between Root-Generated Ionic Currents, pH, Fusicoccin, Indoleacetic Acid, and Growth of the Primary Root of Zea mays. Plant Physiol. 1989 Apr;89(4):1198–1206. doi: 10.1104/pp.89.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey T. J., Evans M. L. Geotropism in corn roots: evidence for its mediation by differential Acid efflux. Science. 1981 Apr 3;212(4490):70–71. doi: 10.1126/science.212.4490.70. [DOI] [PubMed] [Google Scholar]
- Neumann P. M., Van Volkenburgh E., Cleland R. E. Salinity stress inhibits bean leaf expansion by reducing turgor, not wall extensibility. Plant Physiol. 1988;88:233–237. doi: 10.1104/pp.88.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentenac H., Grignon C. Effect of H Excretion on the Surface pH of Corn Root Cells Evaluated by Using Weak Acid Influx as a pH Probe. Plant Physiol. 1987 Aug;84(4):1367–1372. doi: 10.1104/pp.84.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]


