Abstract
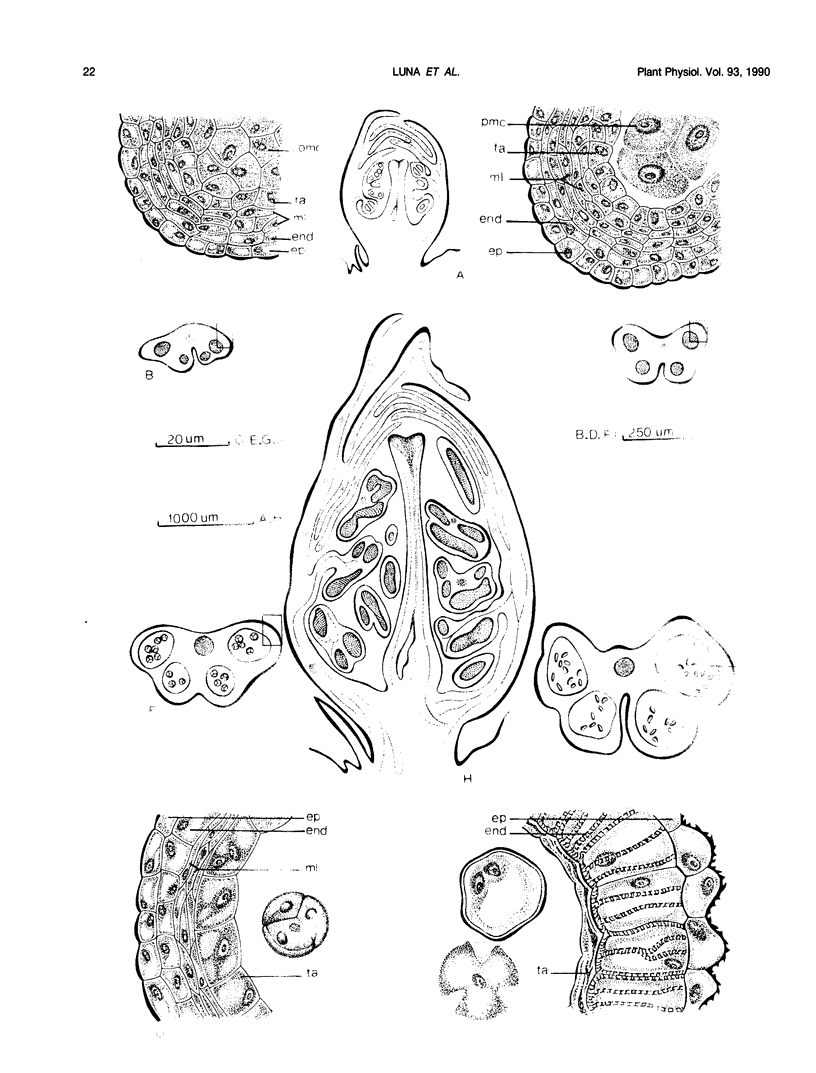
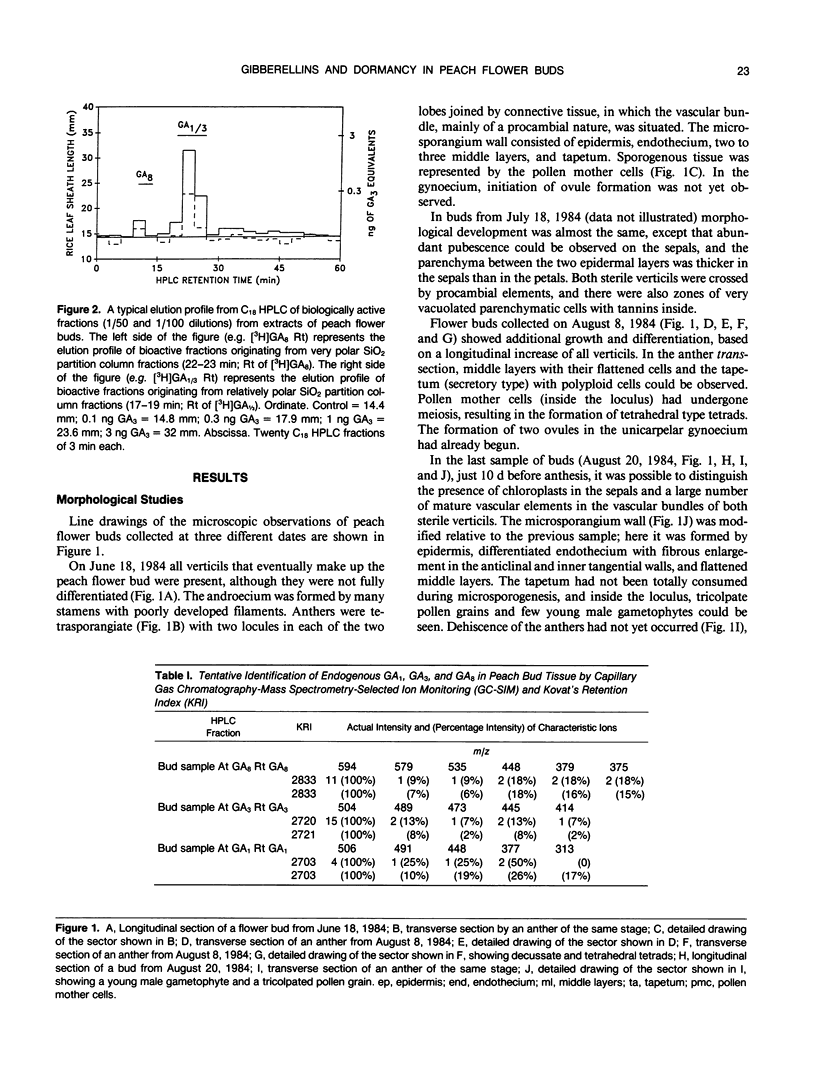
Flower buds of peach (Prunus persica L.) trees, cv Novedad de Cordoba (Argentina), were collected near the end of the dormant period and immediately before anthesis. After removal of scale leaves, morphological observations of representative buds, made on transverse and longitudinal microtome sections, showed that all verticils making up the flower are present in an undifferentiated form during the dormant period (June). Flower buds collected at the end of dormant period (August) showed additional growth and differentiation, at which time formation of two ovules was beginning in the unicarpelar gynoecium. Dehiscence of anthers had not yet occurred 10 days before full bloom, and the ovules were still developing. Free endogenous gibberellin (GA)-like substances were quantified by bioassay (Tan-ginbozu dwarf rice microdrop) after SiO2 partition column chromatography, reversed phase C18-high performance liquid chromatography, and finally Nucleosil [N(CH3)2]high performance liquid chromatography. Bioactive fractions were then subjected to capillary gas chromatography-mass spectrometry-selected ion monitoring (GC-MS-SIM). Gibberellins A1, A3, and A8 were tentatively identified in peach flower buds using GC-SIM and Kovat's retention indices, and relative amounts approximated by GC-SIM (2:8:6 for GA1, GA3, and GA8, respectively). The highest concentration (330 nanograms per gram dry weight) of free GA1/GA3 was found in dormant buds (June) and diminished thereafter. The concentration free of GA1/GA3 did not increase immediately prior to bud break. However, high GA1/GA3 concentrations occurred during stages where rate of growth and cellular differentiation of (mainly fertile) verticils can be influenced.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottini R., de Bottini G., Koshioka M., Pharis R. P., Coombe B. G. Identification of Gibberellins A(1), A(5), A(29), and A(32) from Immature Seeds of Apricot (Prunus armeniaca L.). Plant Physiol. 1985 Jun;78(2):417–419. doi: 10.1104/pp.78.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe B. G. GA32: A Polar Gibberellin with High Biological Potency. Science. 1971 May 21;172(3985):856–857. doi: 10.1126/science.172.3985.856. [DOI] [PubMed] [Google Scholar]
- Koshioka M., Takeno K., Beall F. D., Pharis R. P. Purification and separation of plant gibberellins from their precursors and glucosyl conjugates. Plant Physiol. 1983 Oct;73(2):398–406. doi: 10.1104/pp.73.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Pharis R. P., Koshioka M. Reversible conjugation of gibberellins in situ in maize. Plant Physiol. 1983 Oct;73(2):340–346. doi: 10.1104/pp.73.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]