Abstract
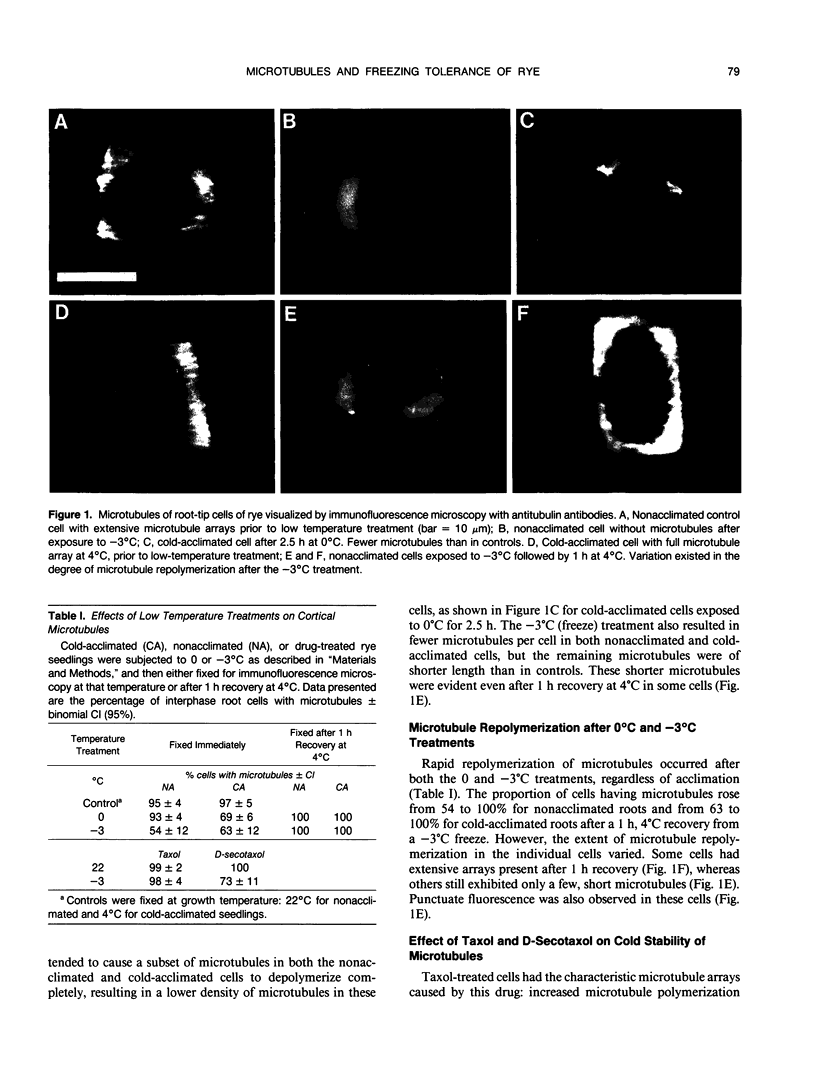
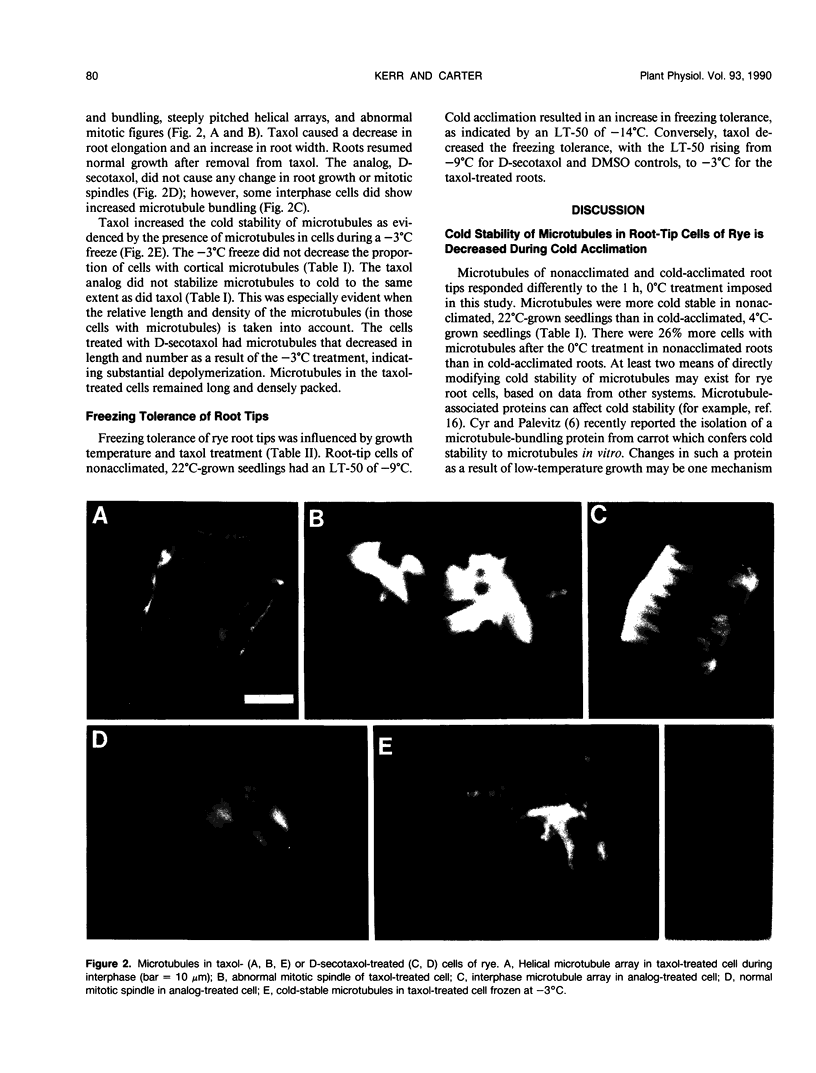
The response of cortical microtubules to low temperature and freezing was assessed for root tips of cold-acclimated and non-acclimated winter rye (Secale cereale L. cv Puma) seedlings using indirect immunofluorescence microscopy with antitubulin antibodies. Roots cooled to 0 or −3°C were fixed for immunofluorescence microscopy at these temperatures or after an additional hour at 4°C. Typical arrays of cortical microtubules were present in root-tip cells of seedlings exposed to the cold-acclimation treatment of 4°C for 2 days. Microtubules in these cold-acclimated cells were more easily depolymerized by a 0°C treatment than microtubules in root-tip cells of nonacclimated, 22°C-grown seedlings. Microtubules were still present in some cells of both nonacclimated and cold-acclimated roots at 0 and −3°C; however, the number of microtubules in these cells was lower than in controls. Microtubules remaining during the −3°C freeze were shorter than microtubules in unfrozen control cells. Repolymerization of microtubules after both the 0 and −3°C treatments occurred within 1 h. Root tips of nonacclimated seedlings had an LT-50 of −9°C. Cold acclimation lowered this value to −14°C. Treatment of 22°C-grown seedlings for 24 h with the microtubule-stabilizing drug taxol caused a decrease in the freezing tolerance of root tips, indicated by a LT-50 of −3°C. Treatment with D-secotaxol, an analog of taxol that was less effective in stabilizing microtubules, did not alter the freezing tolerance. We interpret these data to indicate that a degree of depolymerization of microtubules is necessary for realization of maximum freezing tolerance in root-tip cells of rye.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aszalos A., Yang G. C., Gottesman M. M. Depolymerization of microtubules increases the motional freedom of molecular probes in cellular plasma membranes. J Cell Biol. 1985 May;100(5):1357–1362. doi: 10.1083/jcb.100.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajer A. S., Molè-Bajer J. Asters, poles, and transport properties within spindlelike microtubule arrays. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):263–283. doi: 10.1101/sqb.1982.046.01.029. [DOI] [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Prasad V., Ludueña R. F. Cold-stable microtubules from Antarctic fishes contain unique alpha tubulins. J Biol Chem. 1987 Jun 15;262(17):8360–8366. [PubMed] [Google Scholar]
- Hardham A. R., Gunning B. E. Interpolation of microtubules into cortical arrays during cell elongation and differentiation in roots of Azolla pinnata. J Cell Sci. 1979 Jun;37:411–442. doi: 10.1242/jcs.37.1.411. [DOI] [PubMed] [Google Scholar]
- Hardham A. R., Gunning B. E. Structure of cortical microtubule arrays in plant cells. J Cell Biol. 1978 Apr;77(1):14–34. doi: 10.1083/jcb.77.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel W. A role of microtubules in the polarity of statocytes from roots of Lepidium sativum L. Planta. 1984 Nov;162(5):404–414. doi: 10.1007/BF00393452. [DOI] [PubMed] [Google Scholar]
- Himes R. H., Burton P. R., Gaito J. M. Dimethyl sulfoxide-induced self-assembly of tubulin lacking associated proteins. J Biol Chem. 1977 Sep 10;252(17):6222–6228. [PubMed] [Google Scholar]
- Job D., Rauch C. T., Fischer E. H., Margolis R. L. Recycling of cold-stable microtubules: evidence that cold stability is due to substoichiometric polymer blocks. Biochemistry. 1982 Feb 2;21(3):509–515. doi: 10.1021/bi00532a015. [DOI] [PubMed] [Google Scholar]
- Kerr G. P., Carter J. V. Tubulin Isotypes in Rye Roots Are Altered during Cold Acclimation. Plant Physiol. 1990 May;93(1):83–88. doi: 10.1104/pp.93.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Piperno G., LeDizet M., Chang X. J. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol. 1987 Feb;104(2):289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikin A., Atsmon D., Gitler C. Quantitation of Chill-Induced Release of a Tubulin-Like Factor and Its Prevention by Abscisic Acid in Gossypium hirsutum L. Plant Physiol. 1983 Apr;71(4):747–748. doi: 10.1104/pp.71.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick S. M., Duniec J. Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. I. Preprophase band development and concomitant appearance of nuclear envelope-associated tubulin. J Cell Biol. 1983 Jul;97(1):235–243. doi: 10.1083/jcb.97.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]