Abstract
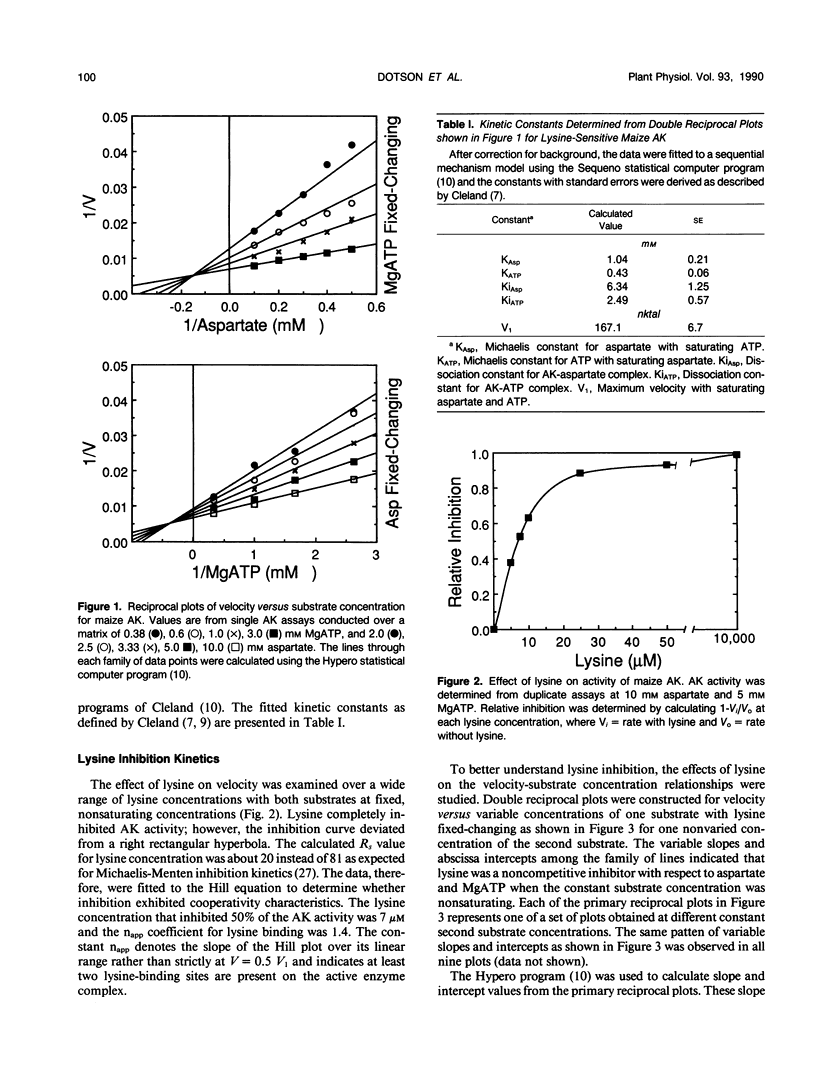
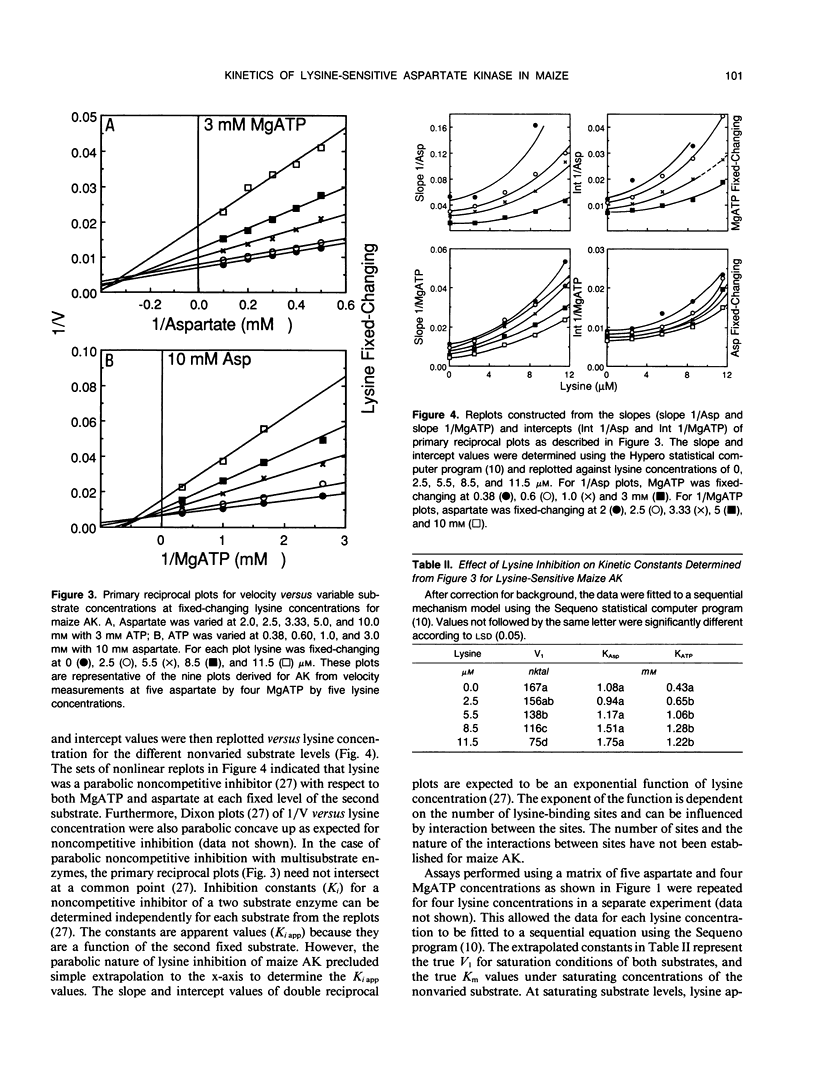
Steady state substrate kinetics and feedback regulation properties were determined for lysine-sensitive aspartate kinase (AK) purified from Black Mexican Sweet maize (Zea mays L.) cell suspension cultures. Two AK isoforms (AK Early and AK Late) were separated by two passages through an anion exchange column as the final steps in a procedure giving 1200-fold purification. Kinetic properties were determined for the major AK Late eluting isoform. Assays were conducted at the pH activity maximum (8.0) and with excess Mg2+ to favor a two-substrate reaction involving aspartate and complexed MgATP. AK catalyzed a sequential reaction in which MgATP and aspartate both bind to the enzyme complex before the ADP and aspartyl-phosphate products are released. The Km value calculated for MgATP was 0.43 millimolar and for aspartate was 1.04 millimolar. Cooperativity in substrate binding was not observed and was not induced by lysine. The lysine concentration required for 50% inhibition of AK activity was 7 micromolar. An apparent Hill coefficient of 1.4 indicated a minimum of two lysine-binding sites on the active AK complex. At nonsaturating substrate concentrations, lysine inhibition was characteristic of an S-parabolic, I-parabolic noncompetitive allosteric inhibitor. The parabolic inhibitor replot, Hill coefficients > 1, and the lack of substrate cooperativity were consistent with a model for multiple lysine-binding sites per active AK subunit. Similar kinetic properties were observed for the AK Early isoform.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bearer C. F., Neet K. E. Threonine inhibition of the aspartokinase--homoserine dehydrogenase I of Escherichia coli. A slow transient and cooperativity of inhibition of the aspartokinase activity. Biochemistry. 1978 Aug 22;17(17):3523–3530. doi: 10.1021/bi00610a016. [DOI] [PubMed] [Google Scholar]
- Beevers H. Conceptual developments in metabolic control, 1924-1974. Plant Physiol. 1974 Oct;54(4):437–442. doi: 10.1104/pp.54.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan P. A., Cawley R. D., Brunner C. E., Bryan J. K. Isolation and characterization of a lysine-sensitive aspartokinase from a multicellular plant. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1211–1217. doi: 10.1016/0006-291x(70)90215-9. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Dotson S. B., Somers D. A., Gengenbach B. G. Purification and characterization of lysine-sensitive aspartate kinase from maize cell cultures. Plant Physiol. 1989 Dec;91(4):1602–1608. doi: 10.1104/pp.91.4.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Aspartokinase of Lemna paucicostata Hegelm. 6746. Plant Physiol. 1989 Aug;90(4):1577–1583. doi: 10.1104/pp.90.4.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R. A., Das P. K., Widholm J. M. Characterization of cultured tobacco cell lines resistant to ethionine, a methionine analog. Plant Physiol. 1984 Mar;74(3):640–644. doi: 10.1104/pp.74.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd K. A., Green C. E. Inheritance and expression of lysine plus threonine resistance selected in maize tissue culture. Proc Natl Acad Sci U S A. 1982 Jan;79(2):559–563. doi: 10.1073/pnas.79.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtley M. E., Koshland D. E., Jr Models for cooperative effects in proteins containing subunits. Effects of two interacting ligands. J Biol Chem. 1967 Sep 25;242(18):4192–4205. [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Rognes S. E., Lea P. J., Miflin B. J. S-adenosylmethionine--a novel regulator of aspartate kinase. Nature. 1980 Sep 25;287(5780):357–359. doi: 10.1038/287357a0. [DOI] [PubMed] [Google Scholar]
- Shewry P. R., Miflin B. J. Properties and Regulation of Aspartate Kinase from Barley Seedlings (Hordeum vulgare L.). Plant Physiol. 1977 Jan;59(1):69–73. doi: 10.1104/pp.59.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wampler D. E., Westhead E. W. Two aspartokinases from Escherichia coli. Nature of the inhibition and molecular changes accompanying reversible inactivation. Biochemistry. 1968 May;7(5):1661–1671. doi: 10.1021/bi00845a007. [DOI] [PubMed] [Google Scholar]


