Abstract
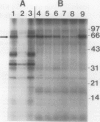
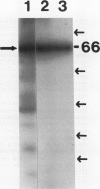
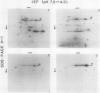
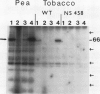
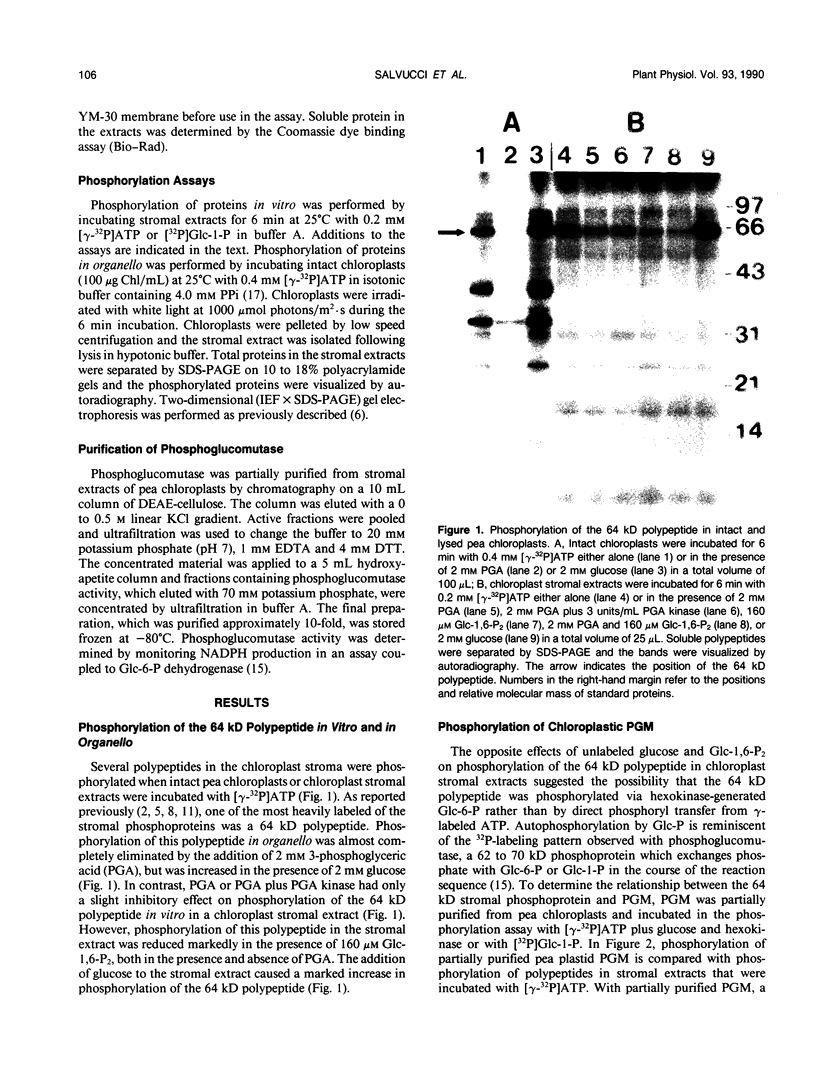
Phosphorylation of the 64 kilodalton stromal phosphoprotein by incubation of pea (Pisum sativum) chloroplast extracts with [γ-32P]ATP decreased in the presence of Glc-6-P and Glc-1,6-P2, but was stimulated by glucose. Two-dimensional gel electrophoresis following incubation of intact chloroplasts and stromal extracts with [γ-32P]ATP, or incubation of stromal extracts and partially purified phosphoglucomutase (EC 2.7.5.1) with [32P]Glc-1-P showed that the identical 64 kilodalton polypeptide was labeled. A 62 kilodalton polypeptide was phosphorylated by incubation of tobacco (Nicotiana sylvestris) stromal extracts with either [γ-32P]ATP or [32P]Glc-1-P. In contrast, an analogous polypeptide was not phosphorylated in extracts from a tobacco mutant deficient in plastid phosphoglucomutase activity. The results indicate that the 64 (or 62) kilodalton chloroplast stromal phosphoprotein is phosphoglucomutase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhalla P., Bennett J. Chloroplast phosphoproteins: phosphorylation of a 12-kDa stromal protein by the redox-controlled kinase of thylakoid membranes. Arch Biochem Biophys. 1987 Jan;252(1):97–104. doi: 10.1016/0003-9861(87)90012-9. [DOI] [PubMed] [Google Scholar]
- Cortez N., Lucero H. A., Vallejos R. H. Stromal serine protein kinase activity in spinach chloroplasts. Arch Biochem Biophys. 1987 May 1;254(2):504–508. doi: 10.1016/0003-9861(87)90130-5. [DOI] [PubMed] [Google Scholar]
- Drake R. R., Jr, Evans R. K., Wolf M. J., Haley B. E. Synthesis and properties of 5-azido-UDP-glucose. Development of photoaffinity probes for nucleotide diphosphate sugar binding sites. J Biol Chem. 1989 Jul 15;264(20):11928–11933. [PubMed] [Google Scholar]
- Foyer C. H. Stromal protein phosphorylation in spinach (Spinacia oleracea) chloroplasts. Biochem J. 1985 Oct 1;231(1):97–103. doi: 10.1042/bj2310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitton C., Mache R. Phosphorylation in vitro of the large subunit of the ribulose-1,5-bisphosphate carboxylase and of the glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1987 Jul 1;166(1):249–254. doi: 10.1111/j.1432-1033.1987.tb13509.x. [DOI] [PubMed] [Google Scholar]
- Hanson K. R., McHale N. A. A Starchless Mutant of Nicotiana sylvestris Containing a Modified Plastid Phosphoglucomutase. Plant Physiol. 1988 Nov;88(3):838–844. doi: 10.1104/pp.88.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter R. L., Haley B. E. Photoaffinity labeling of nucleotide binding sites with 8-azidopurine analogs: techniques and applications. Methods Enzymol. 1983;91:613–633. doi: 10.1016/s0076-6879(83)91054-6. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Portis A. R. Involvement of stromal ATP in the light activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in intact isolated chloroplasts. Plant Physiol. 1988 Jan;86(1):293–298. doi: 10.1104/pp.86.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Pyrophosphate inhibition of carbon dioxide fixation in isolated pea chloroplasts by uptake in exchange for endogenous adenine nucleotides. Plant Physiol. 1977 Mar;59(3):422–427. doi: 10.1104/pp.59.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J., Bennett J. Localization of a 64-kDa phosphoprotein in the lumen between the outer and inner envelopes of pea chloroplasts. Eur J Biochem. 1988 Aug 1;175(2):301–307. doi: 10.1111/j.1432-1033.1988.tb14197.x. [DOI] [PubMed] [Google Scholar]