Abstract
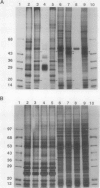
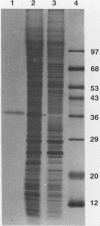
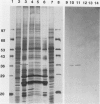
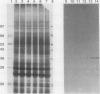
Chlamydomonas reinhardtii possesses a CO2-concentrating mechanism, induced by limiting CO2, which involves active transport and accumulation of inorganic carbon within the cell. Synthesis of several proteins is induced by limiting CO2, but, of those, only periplasmic carbonic anhydrase has an identified function in the system. No proteins involved in active transport have yet been identified, but induced, membrane-associated polypeptides, such as the 36 kilodalton polypeptide focused on in this paper, would seem to be candidates for such involvement. The 36 kilodalton polypeptide was shown to be synthesized de novo upon transfer of cells to limiting CO2. It was purified using SDS-PAGE and used to produce polyclonal antibodies. Antibodies were used to confirm the air-specific nature of the polypeptide, its strict association with membrane fractions, and the time course of its induction. Using the antibodies, a single, 36 kilodalton polypeptide was found to be specifically immunoprecipitated from in vitro translation products of poly(A+) RNA from cells only after exposure to limiting CO2. The absence of translatable mRNA for this polypeptide in CO2-enriched cells indicated that regulation occurs at the level of message abundance. The antibodies were also used to demonstrate the distinction between the limiting-CO2 induced 36 kilodalton polypeptide and the similarly sized, limiting-CO2 induced periplasmic carbonic anhydrase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba S., Mishima H., Miyachi Y. Levels of cyclic-AMP, cyclic-GMP and betamethasone in the aqueous humor following topical administration of betamethasone in rabbit eyes. Hiroshima J Med Sci. 1983 Sep;32(3):301–304. [PubMed] [Google Scholar]
- Bailly J., Coleman J. R. Effect of CO(2) Concentration on Protein Biosynthesis and Carbonic Anhydrase Expression in Chlamydomonas reinhardtii. Plant Physiol. 1988 Aug;87(4):833–840. doi: 10.1104/pp.87.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert J. T., Hershey H. P., Quail P. H. Autoregulatory control of translatable phytochrome mRNA levels. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2248–2252. doi: 10.1073/pnas.80.8.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Berry J. A., Togasaki R. K., Grossman A. R. Identification of Extracellular Carbonic Anhydrase of Chlamydomonas reinhardtii. Plant Physiol. 1984 Oct;76(2):472–477. doi: 10.1104/pp.76.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Grossman A. R. Biosynthesis of carbonic anhydrase in Chlamydomonas reinhardtii during adaptation to low CO(2). Proc Natl Acad Sci U S A. 1984 Oct;81(19):6049–6053. doi: 10.1073/pnas.81.19.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manuel L. J., Moroney J. V. Inorganic Carbon Accumulation by Chlamydomonas reinhardtii: New Proteins are made During Adaptation to Low CO(2). Plant Physiol. 1988 Oct;88(2):491–496. doi: 10.1104/pp.88.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T., Ogawa T. Biosynthesis of a 42-kD Polypeptide in the Cytoplasmic Membrane of the Cyanobacterium Anacystis nidulans Strain R2 during Adaptation to Low CO(2) Concentration. Plant Physiol. 1986 Feb;80(2):525–530. doi: 10.1104/pp.80.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist K., Sjöberg S., Samuelsson G. Induction of Inorganic Carbon Accumulation in the Unicellular Green Algae Scenedesmus obliquus and Chlamydomonas reinhardtii. Plant Physiol. 1988 Jun;87(2):437–442. doi: 10.1104/pp.87.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Fluorographic detection of radioactivity in polyacrylamide gels with 2,5-diphenyloxazole in acetic acid and its comparison with existing procedures. Biochem J. 1983 Jan 1;209(1):281–284. doi: 10.1042/bj2090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Jeffrey M. Membrane-Associated Polypeptides Induced in Chlamydomonas by Limiting CO(2) Concentrations. Plant Physiol. 1989 Jan;89(1):133–137. doi: 10.1104/pp.89.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]