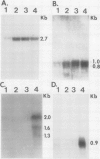
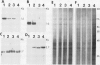
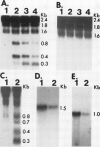
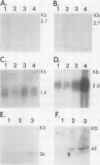
Abstract
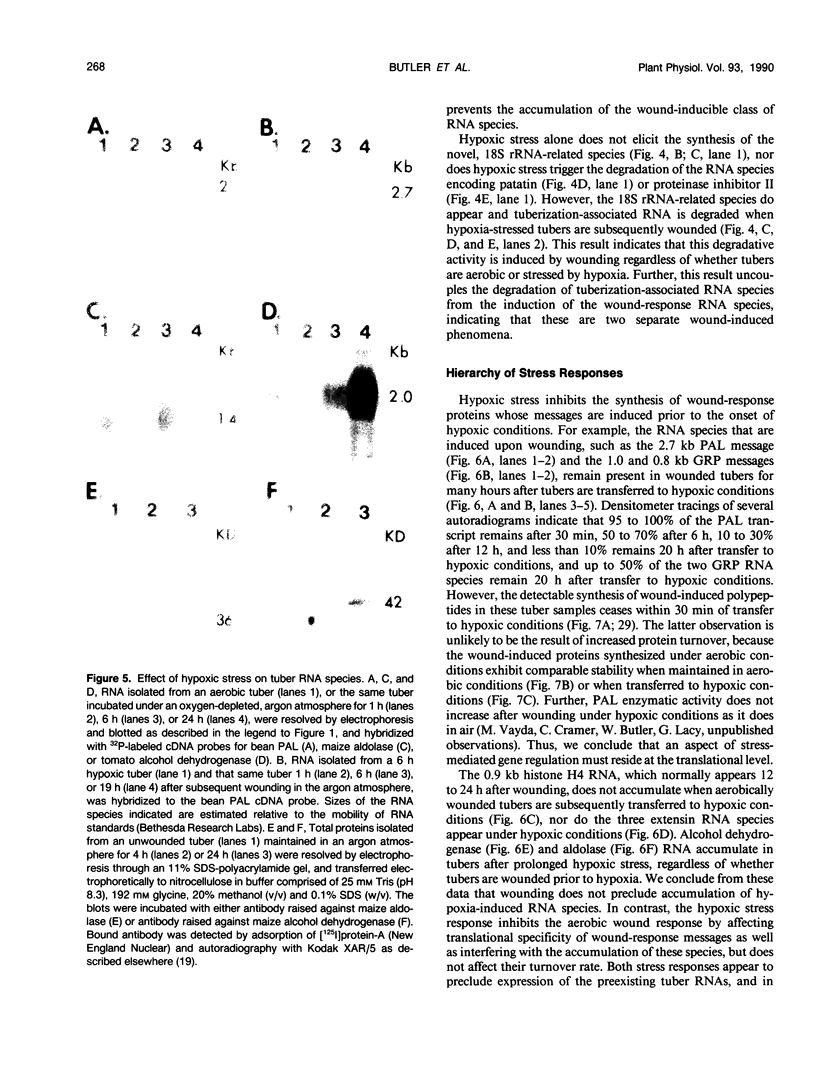
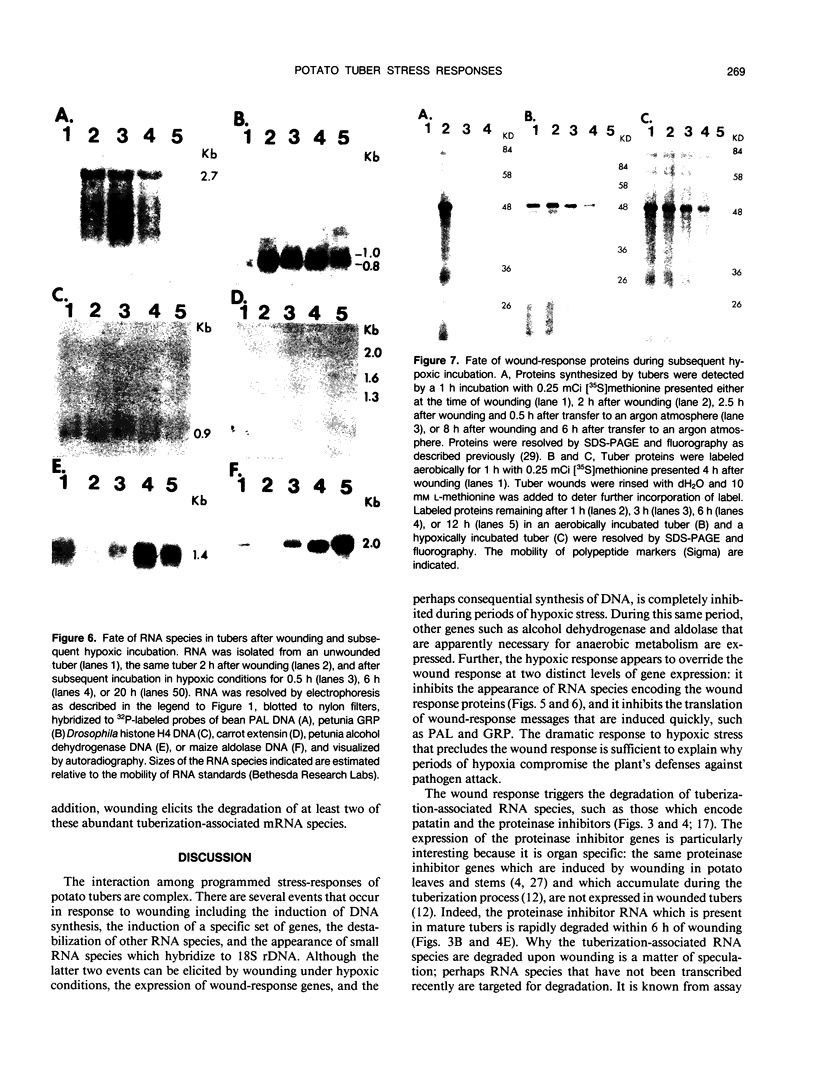
Potato (Solanum tuberosum L.) tubers subjected to wounding under hypoxic stress do not synthesize RNA species that are induced in response to wounding in aerobic conditions. Further, wound-response proteins fail to be synthesized when wounded tubers are transferred to hypoxic conditions although messenger RNAs which encode them persist for many hours after transfer. Hypoxic stress also prevents the incorporation of [3H]thymidine by wounded tubers that occurs in aerobic conditions. In contrast, hypoxic tubers accumulate and translate transcripts of genes whose products are involved in anaerobic metabolism whether or not they are wounded. Both the hypoxic response and the aerobic wound response preclude the synthesis of proteins encoded by messenger RNAs which accumulated during the tuberization process and which can be translated in vitro. Finally, wounding elicits the degradation of a subset of these tuberization-associated transcripts. These data indicate a complex and precise regulation of gene expression at several levels of macromolecular synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen J., Varner J. E. Isolation and characterization of cDNA clones for carrot extensin and a proline-rich 33-kDa protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4399–4403. doi: 10.1073/pnas.82.13.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit C. M., Meagher R. B. Expression of a gene encoding a glycine-rich protein in petunia. Mol Cell Biol. 1987 Dec;7(12):4273–4279. doi: 10.1128/mcb.7.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K., Cramer C. L., Bolwell G. P., Dixon R. A., Schuch W., Lamb C. J. Rapid transient induction of phenylalanine ammonia-lyase mRNA in elicitor-treated bean cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6731–6735. doi: 10.1073/pnas.82.20.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. S., Pearce G., Merryweather J., Titani K., Ericsson L. H., Ryan C. A. Wound-induced proteinase inhibitors from tomato leaves. II. The cDNA-deduced primary structure of pre-inhibitor II. J Biol Chem. 1985 Jun 10;260(11):6561–6564. [PubMed] [Google Scholar]
- Hake S., Kelley P. M., Taylor W. C., Freeling M. Coordinate induction of alcohol dehydrogenase 1, aldolase, and other anaerobic RNAs in maize. J Biol Chem. 1985 Apr 25;260(8):5050–5054. [PubMed] [Google Scholar]
- Hightower R. C., Meagher R. B. Divergence and differential expression of soybean actin genes. EMBO J. 1985 Jan;4(1):1–8. doi: 10.1002/j.1460-2075.1985.tb02309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil M., Sánchez-Serrano J. J., Willmitzer L. Both wound-inducible and tuber-specific expression are mediated by the promoter of a single member of the potato proteinase inhibitor II gene family. EMBO J. 1989 May;8(5):1323–1330. doi: 10.1002/j.1460-2075.1989.tb03512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller B., Sauer N., Lamb C. J. Glycine-rich cell wall proteins in bean: gene structure and association of the protein with the vascular system. EMBO J. 1988 Dec 1;7(12):3625–3633. doi: 10.1002/j.1460-2075.1988.tb03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Tolan D. R. The complete amino Acid sequence for the anaerobically induced aldolase from maize derived from cDNA clones. Plant Physiol. 1986 Dec;82(4):1076–1080. doi: 10.1104/pp.82.4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Hahlbrock K., Lamb C. Rapid induction of the synthesis of phenylalanine ammonia-lyase and of chalcone synthase in elicitor-treated plant cells. Eur J Biochem. 1983 Jan 1;129(3):593–601. doi: 10.1111/j.1432-1033.1983.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J., Lipphardt S., Lörz H., Häuser I., Willmitzer L., Schell J. 5' upstream sequences from the wun1 gene are responsible for gene activation by wounding in transgenic plants. Plant Cell. 1989 Jan;1(1):151–158. doi: 10.1105/tpc.1.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J., Mayer J. E., Schell J., Willmitzer L. Differential expression of genes in potato tubers after wounding. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1136–1140. doi: 10.1073/pnas.85.4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt R., Vayda M. E., Young M., Flint S. J. Isolation and characterization of monoclonal antibodies against the adenovirus core proteins. Virology. 1988 May;164(1):275–279. doi: 10.1016/0042-6822(88)90645-9. [DOI] [PubMed] [Google Scholar]
- Mignery G. A., Pikaard C. S., Hannapel D. J., Park W. D. Isolation and sequence analysis of cDNAs for the major potato tuber protein, patatin. Nucleic Acids Res. 1984 Nov 12;12(21):7987–8000. doi: 10.1093/nar/12.21.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva E., Lister R. M., Park W. D. Induction and accumulation of major tuber proteins of potato in stems and petioles. Plant Physiol. 1983 Jan;71(1):161–168. doi: 10.1104/pp.71.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto J. E., Suzich J. A., Herrmann K. M. 3-Deoxy-d-arabino-Heptulosonate 7-Phosphate Synthase from Potato Tuber (Solanum tuberosum L.). Plant Physiol. 1986 Dec;82(4):1040–1044. doi: 10.1104/pp.82.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder T. B., Cramer C. L., Bell J. N., Robbins M. P., Dixon R. A., Lamb C. J. Elicitor rapidly induces chalcone synthase mRNA in Phaseolus vulgaris cells at the onset of the phytoalexin defense response. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5724–5728. doi: 10.1073/pnas.81.18.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Serrano J. J., Keil M., O'Connor A., Schell J., Willmitzer L. Wound expression of a potato proteinase inhibitor II gene in transgenic tobacco plants. EMBO J. 1987 Feb;6(2):303–306. doi: 10.1002/j.1460-2075.1987.tb04754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter A. M., Bell J. N., Cramer C. L., Bailey J. A., Varner J. E., Lamb C. J. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6551–6555. doi: 10.1073/pnas.82.19.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayda M. E., Schaeffer H. J. Hypoxic stress inhibits the appearance of wound-response proteins in potato tubers. Plant Physiol. 1988 Nov;88(3):805–809. doi: 10.1104/pp.88.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]