Abstract
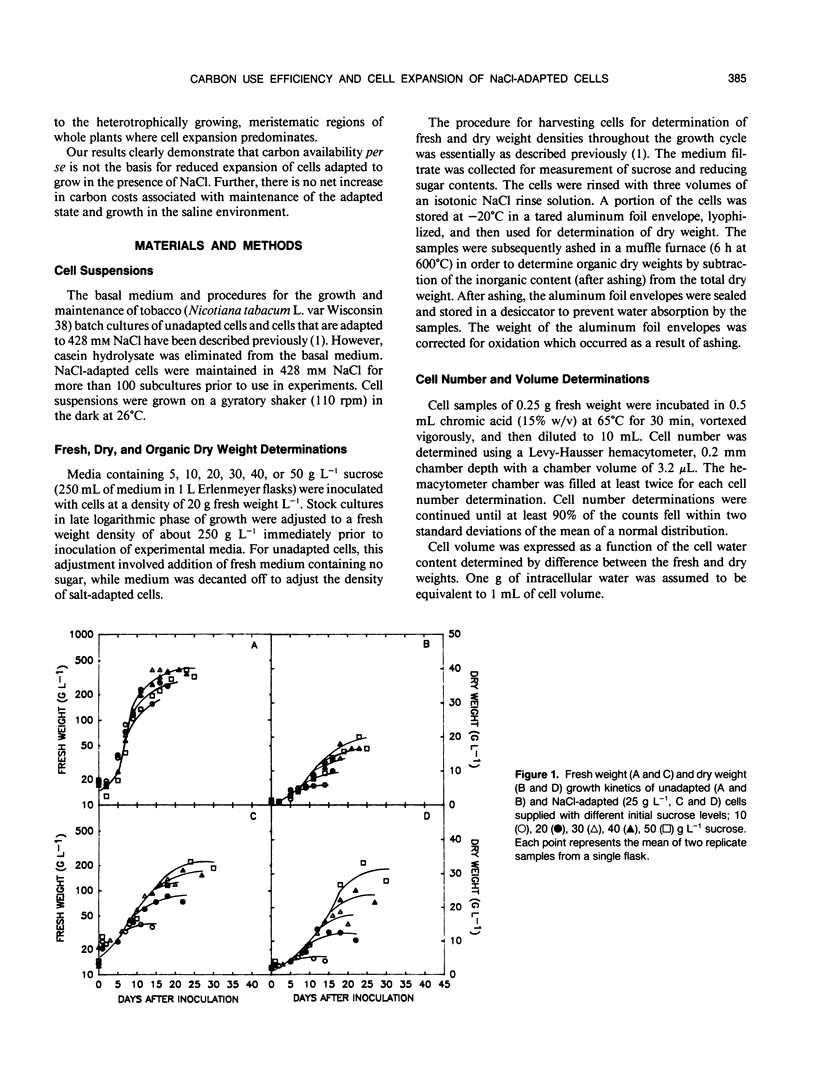
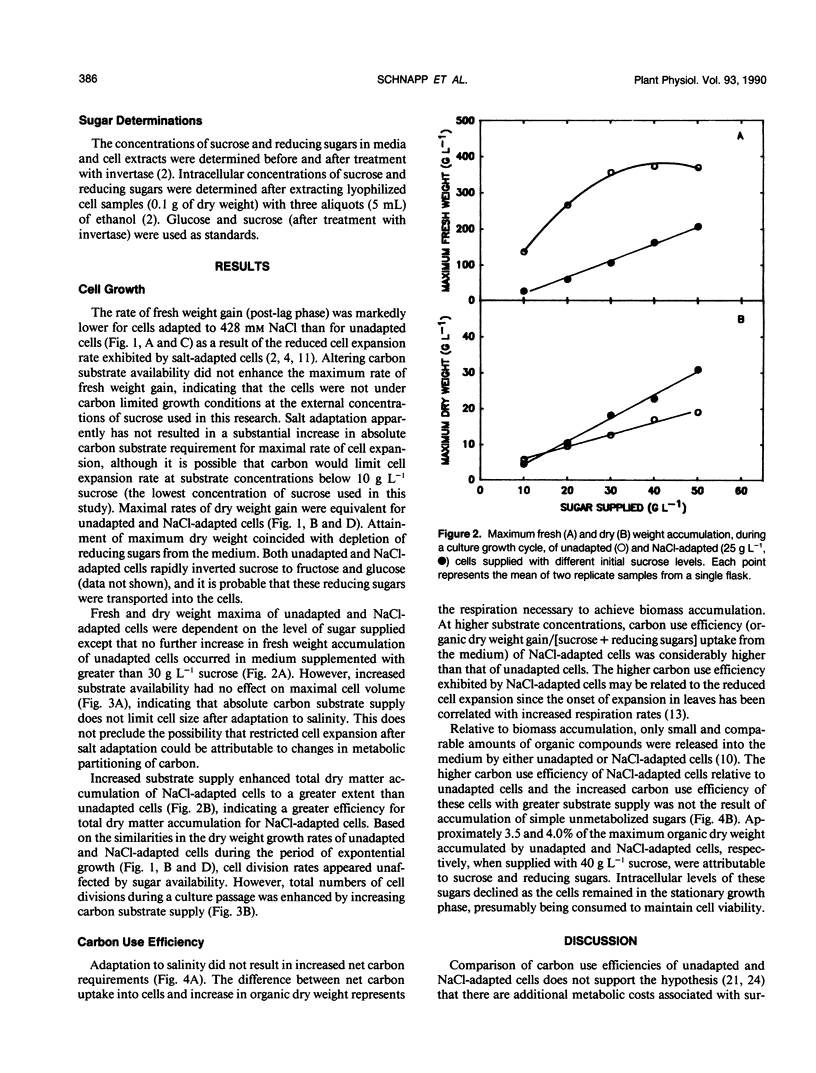
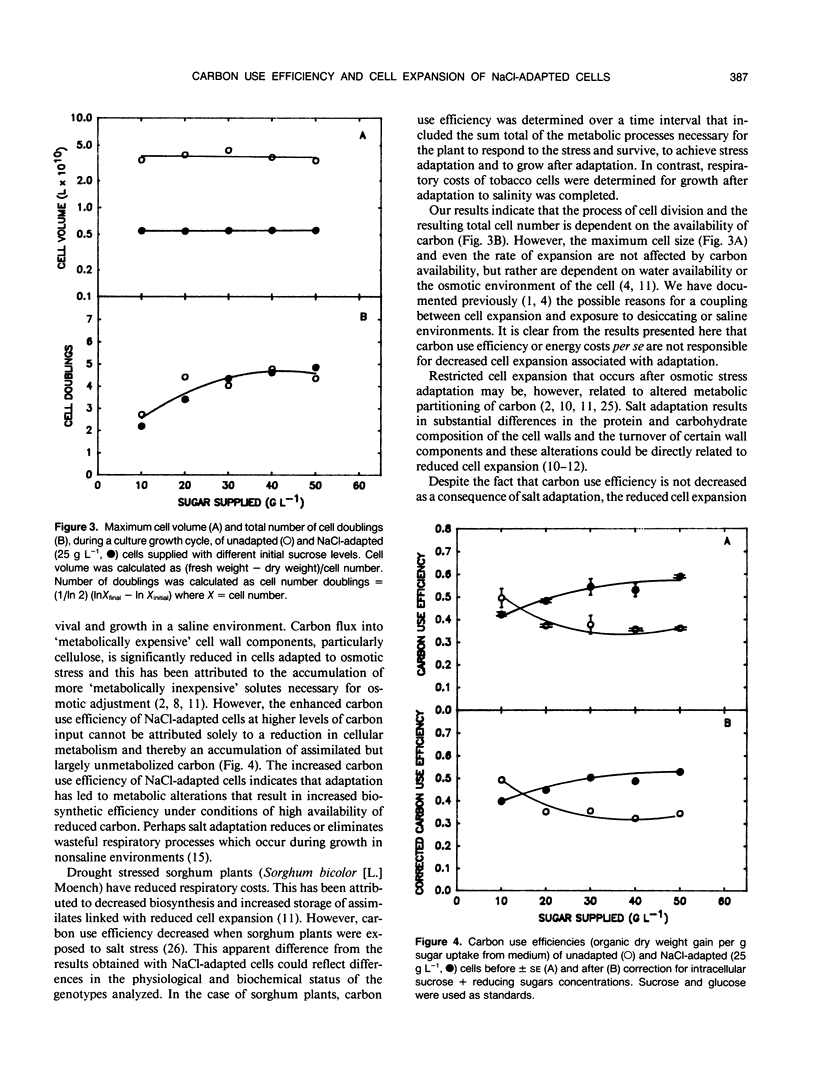
Carbon use efficiencies (gram cell organic dry weight accumulated per gram sugar assimilated from the medium) of unadapted and NaCl-adapted (428 millimolar) cells of tobacco (Nicotiana tabacum L. var Wisconsin 38) were determined to evaluate metabolic costs associated with growth and survival in a saline environment. No net increase in carbon costs was associated with salt adaptation. At low substrate levels, carbon use efficiencies of unadapted and NaCl-adapted cells were not appreciably different (0.495 and 0.422, respectively) and at higher substrate levels carbon use efficiency of NaCl-adapted cells was clearly higher than that of unadapted cells. These results indicate that a homeostasis of metabolic efficiency is established after cells have adapted to NaCl. Altered carbon availability does not cause the reduced cell volume that results from adaptation to NaCl. This does not preclude, however, the possibility that altered intracellular partitioning of carbon affects cell expansion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binzel M. L., Hasegawa P. M., Handa A. K., Bressan R. A. Adaptation of Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):118–125. doi: 10.1104/pp.79.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M. L., Hasegawa P. M., Rhodes D., Handa S., Handa A. K., Bressan R. A. Solute Accumulation in Tobacco Cells Adapted to NaCl. Plant Physiol. 1987 Aug;84(4):1408–1415. doi: 10.1104/pp.84.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M. L., Hess F. D., Bressan R. A., Hasegawa P. M. Intracellular compartmentation of ions in salt adapted tobacco cells. Plant Physiol. 1988 Feb;86(2):607–614. doi: 10.1104/pp.86.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan R. A., Handa A. K., Handa S., Hasegawa P. M. Growth and water relations of cultured tomato cells after adjustment to low external water potentials. Plant Physiol. 1982 Nov;70(5):1303–1309. doi: 10.1104/pp.70.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa S., Bressan R. A., Handa A. K., Carpita N. C., Hasegawa P. M. Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant Physiol. 1983 Nov;73(3):834–843. doi: 10.1104/pp.73.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraki N. M., Bressan R. A., Carpita N. C. Extracellular polysaccharides and proteins of tobacco cell cultures and changes in composition associated with growth-limiting adaptation to water and saline stress. Plant Physiol. 1989 Sep;91(1):54–61. doi: 10.1104/pp.91.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraki N. M., Bressan R. A., Hasegawa P. M., Carpita N. C. Alteration of the physical and chemical structure of the primary cell wall of growth-limited plant cells adapted to osmotic stress. Plant Physiol. 1989 Sep;91(1):39–47. doi: 10.1104/pp.91.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraki N. M., Singh N., Bressan R. A., Carpita N. C. Cell Walls of Tobacco Cells and Changes in Composition Associated with Reduced Growth upon Adaptation to Water and Saline Stress. Plant Physiol. 1989 Sep;91(1):48–53. doi: 10.1104/pp.91.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livne A., Levin N. Tissue Respiration and Mitochondrial Oxidative Phosphorylation of NaCl-Treated Pea Seedlings. Plant Physiol. 1967 Mar;42(3):407–414. doi: 10.1104/pp.42.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCree K. J., Kallsen C. E., Richardson S. G. Carbon Balance of Sorghum Plants during Osmotic Adjustment to Water Stress. Plant Physiol. 1984 Dec;76(4):898–902. doi: 10.1104/pp.76.4.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelena V. A., Boyer J. S. Complete turgor maintenance at low water potentials in the elongating region of maize leaves. Plant Physiol. 1982 May;69(5):1145–1149. doi: 10.1104/pp.69.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. G., McCree K. J. Carbon balance and water relations of sorghum exposed to salt and water stress. Plant Physiol. 1985 Dec;79(4):1015–1020. doi: 10.1104/pp.79.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Sharkey T. D. Salinity and Nitrogen Effects on Photosynthesis, Ribulose-1,5-Bisphosphate Carboxylase and Metabolite Pool Sizes in Phaseolus vulgaris L. Plant Physiol. 1986 Oct;82(2):555–560. doi: 10.1104/pp.82.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termaat A., Passioura J. B., Munns R. Shoot Turgor Does Not Limit Shoot Growth of NaCl-Affected Wheat and Barley. Plant Physiol. 1985 Apr;77(4):869–872. doi: 10.1104/pp.77.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]


