Abstract
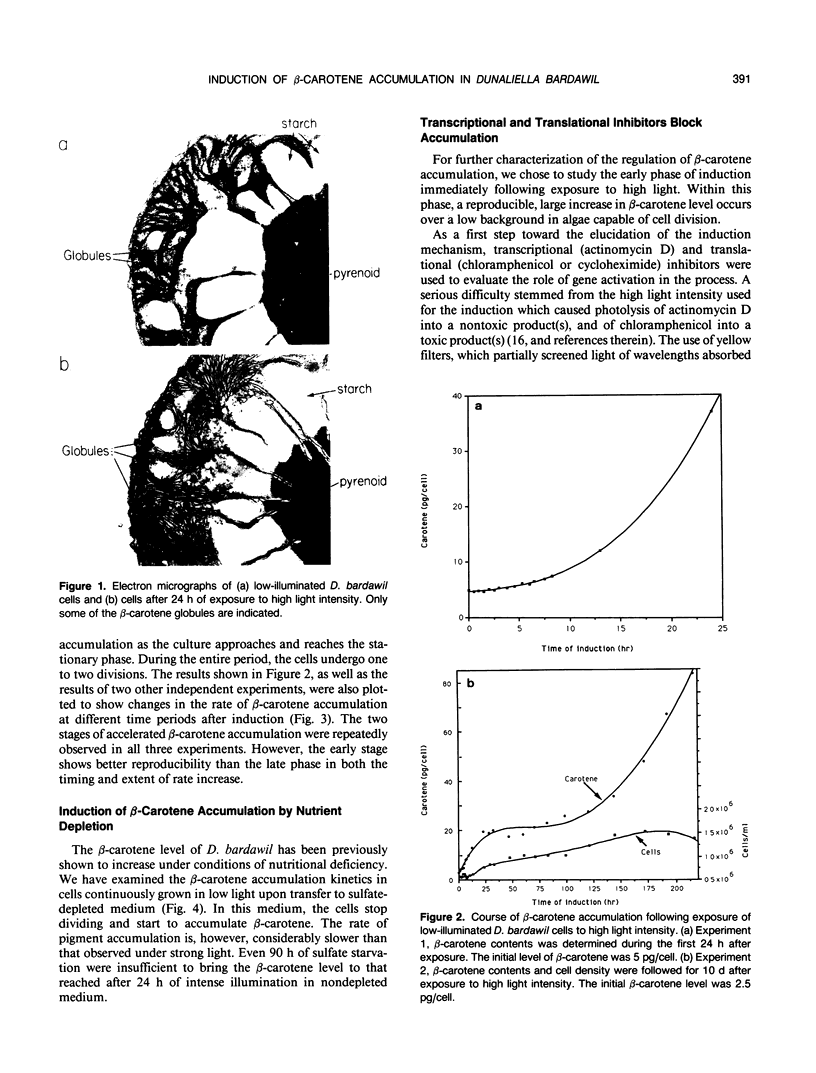
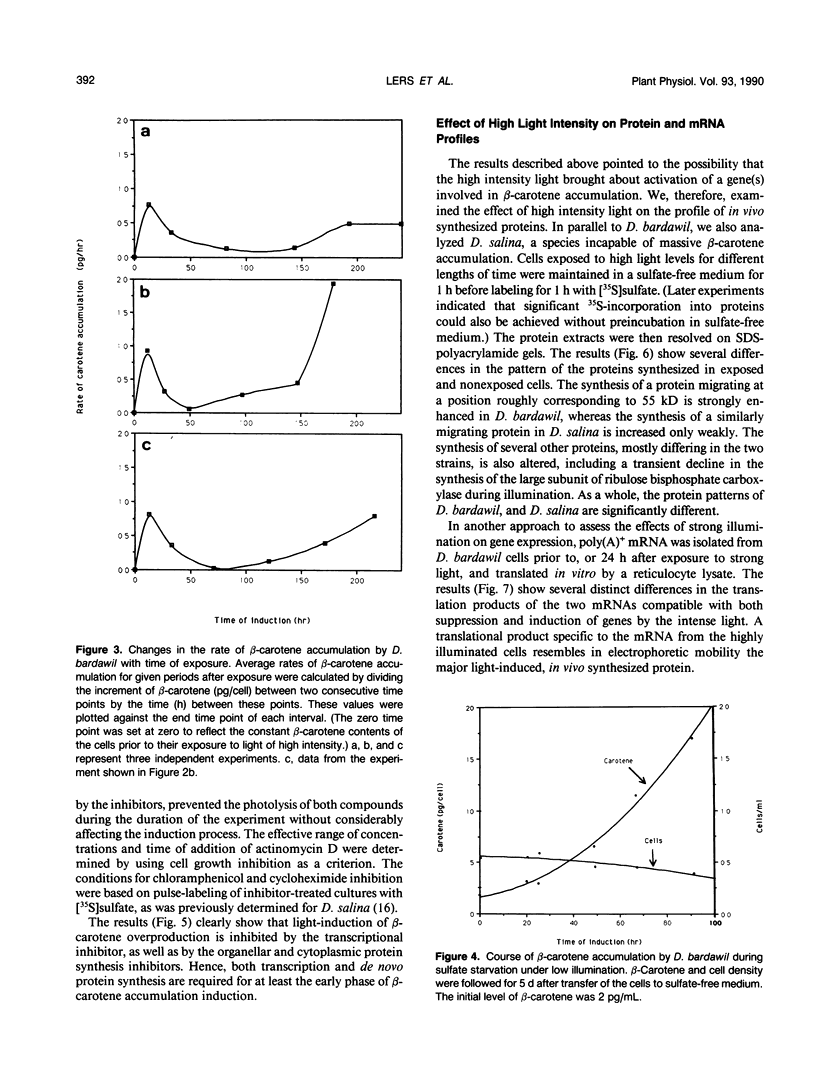
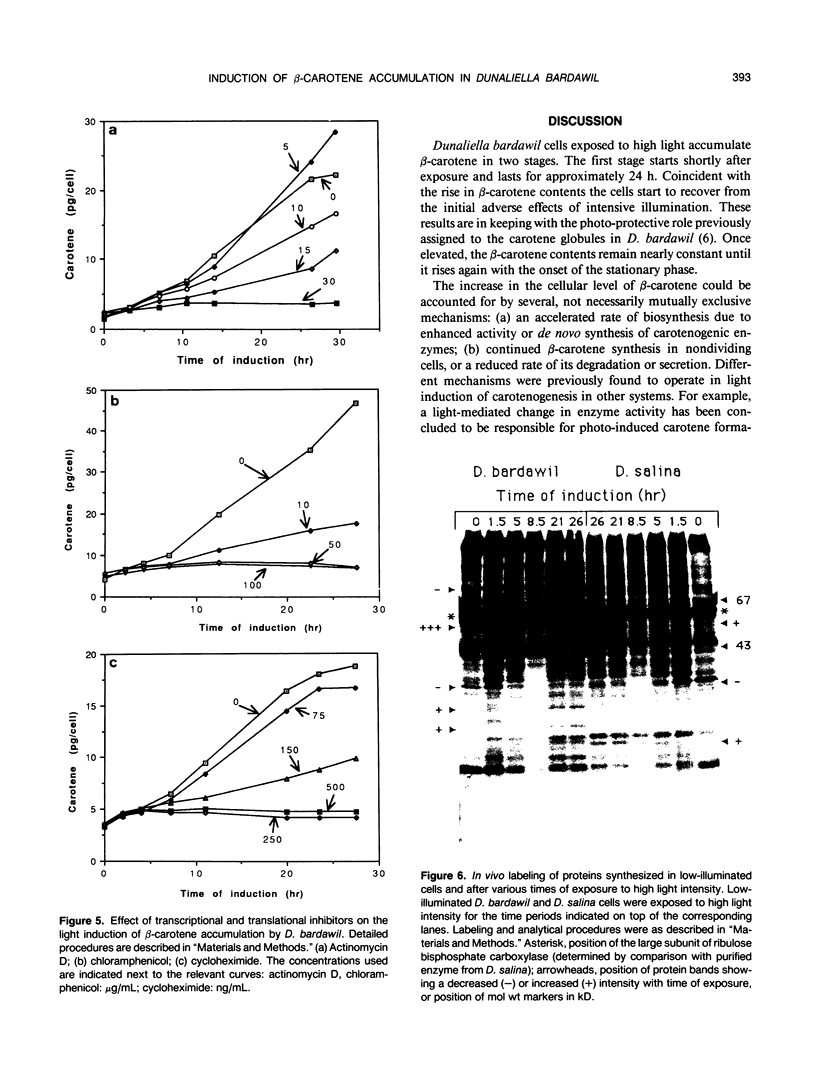
The massive accumulation of β-carotene by the halotolerant micro alga Dunaliella bardawil, in response to high light intensity and several other environmental factors, has been studied so far under different sets of fixed conditions. To determine the kinetics and characteristics of the induction of β-carotene accumulation, cells continuously grown under white light of approximately 27 microeinsteins per square meter per second were exposed to light of approximately 1650 microeinsteins per square meter per second. The exposed cells accumulate β-carotene in two stages: the first stage, lasting for 24 hours, starts shortly after exposure, whereas the second stage starts concomitantly with the onset of the stationary phase and persists until the cells collapse. Actinomycin D, chloramphenicol, or cycloheximide added to low-illuminated cultures abolish the subsequent induction of β-carotene accumulation by high light intensity. These results, together with the early exponential kinetics of accumulation, point to the role of gene activation in the process. In vivo labeling of proteins and in vitro translation of poly(A)+ mRNA revealed several pronounced differences between low-illuminated and high-illuminated cells. A strongly light-induced protein of approximately 55 kilodaltons, as well as other light-induced proteins could possibly fulfill a carotenogenic function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Amotz A., Avron M. On the Factors Which Determine Massive beta-Carotene Accumulation in the Halotolerant Alga Dunaliella bardawil. Plant Physiol. 1983 Jul;72(3):593–597. doi: 10.1104/pp.72.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A., Lers A., Avron M. Stereoisomers of beta-Carotene and Phytoene in the Alga Dunaliella bardawil. Plant Physiol. 1988 Apr;86(4):1286–1291. doi: 10.1104/pp.86.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley P. M., Mackenzie A. Regulation of carotenoid biosynthesis. Curr Top Cell Regul. 1988;29:291–343. doi: 10.1016/b978-0-12-152829-4.50009-4. [DOI] [PubMed] [Google Scholar]
- Harding R. W., Turner R. V. Photoregulation of the Carotenoid Biosynthetic Pathway in Albino and White Collar Mutants of Neurospora crassa. Plant Physiol. 1981 Sep;68(3):745–749. doi: 10.1104/pp.68.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Logemann J., Schell J., Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987 May 15;163(1):16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Nelson M. A., Morelli G., Carattoli A., Romano N., Macino G. Molecular cloning of a Neurospora crassa carotenoid biosynthetic gene (albino-3) regulated by blue light and the products of the white collar genes. Mol Cell Biol. 1989 Mar;9(3):1271–1276. doi: 10.1128/mcb.9.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Greene F. C. Wheat Storage Proteins : ISOLATION AND CHARACTERIZATION OF THE GLIADIN MESSENGER RNAs. Plant Physiol. 1982 Apr;69(4):834–839. doi: 10.1104/pp.69.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]