Abstract
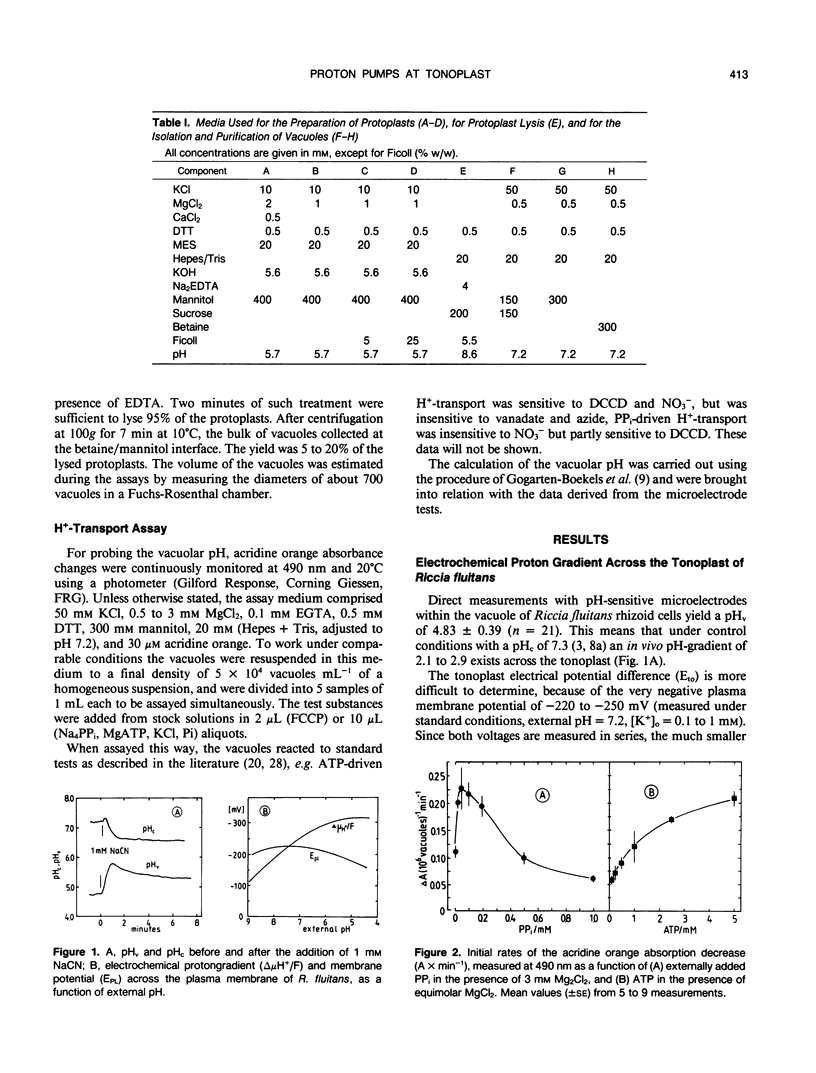
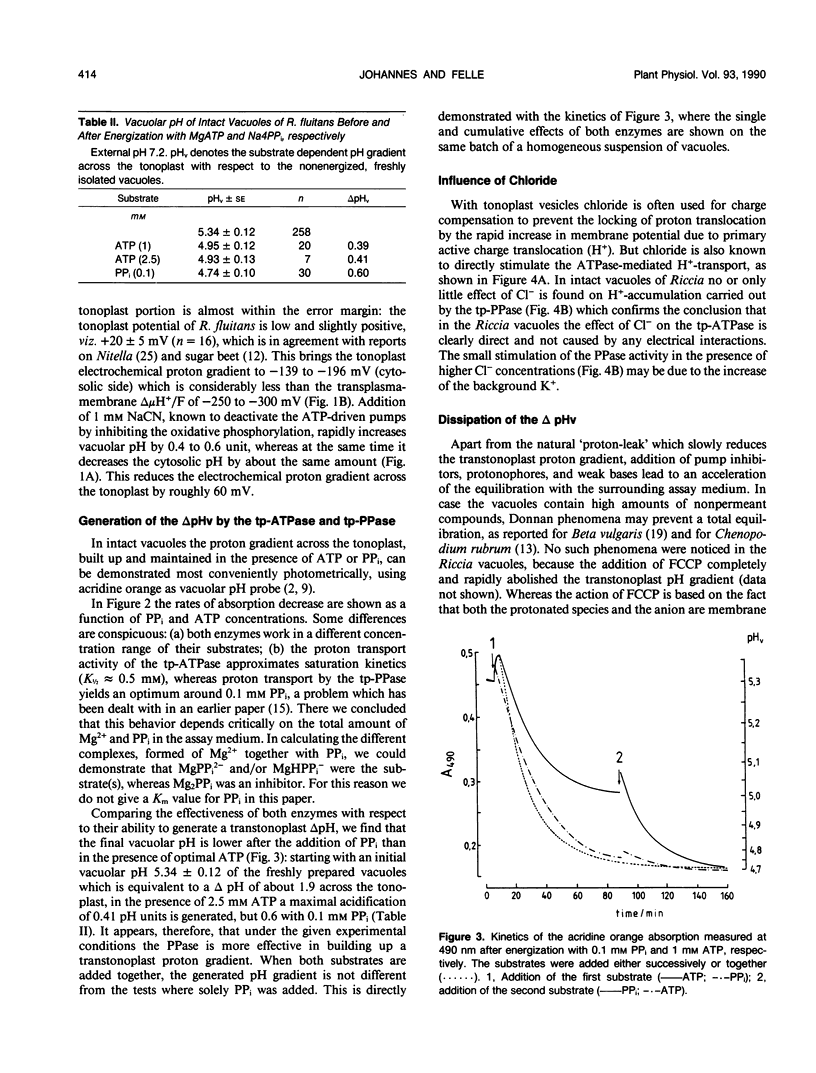
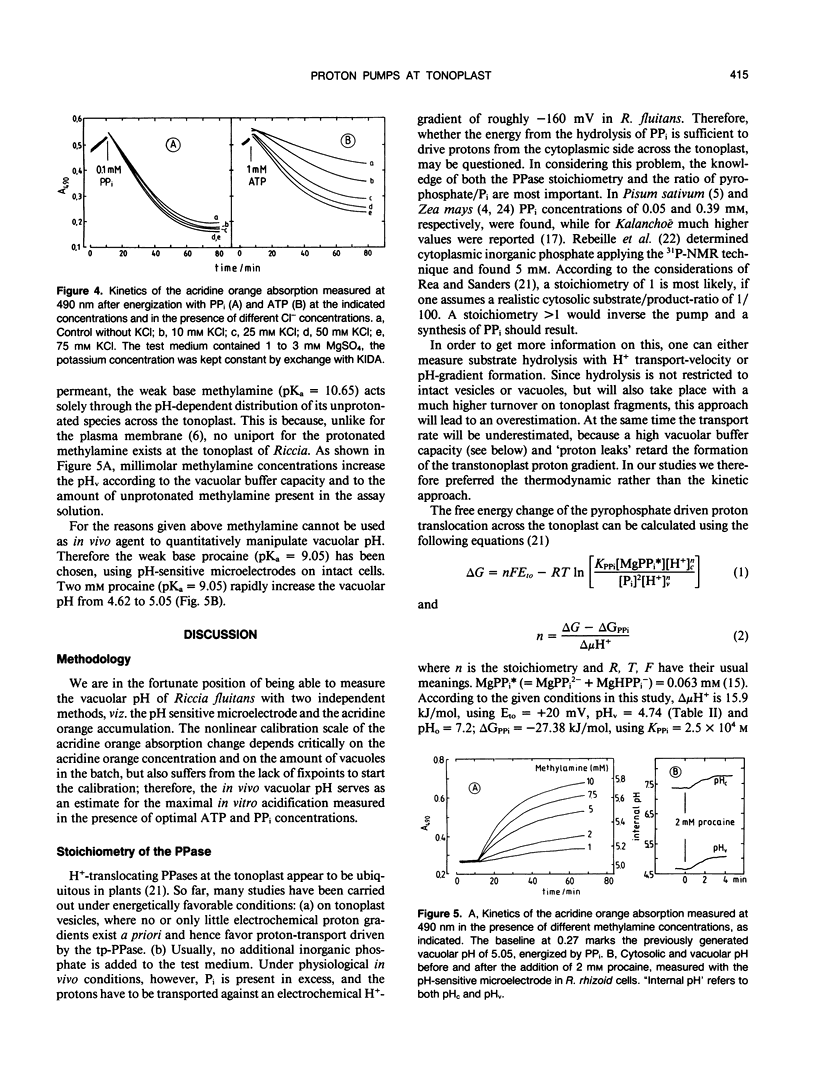
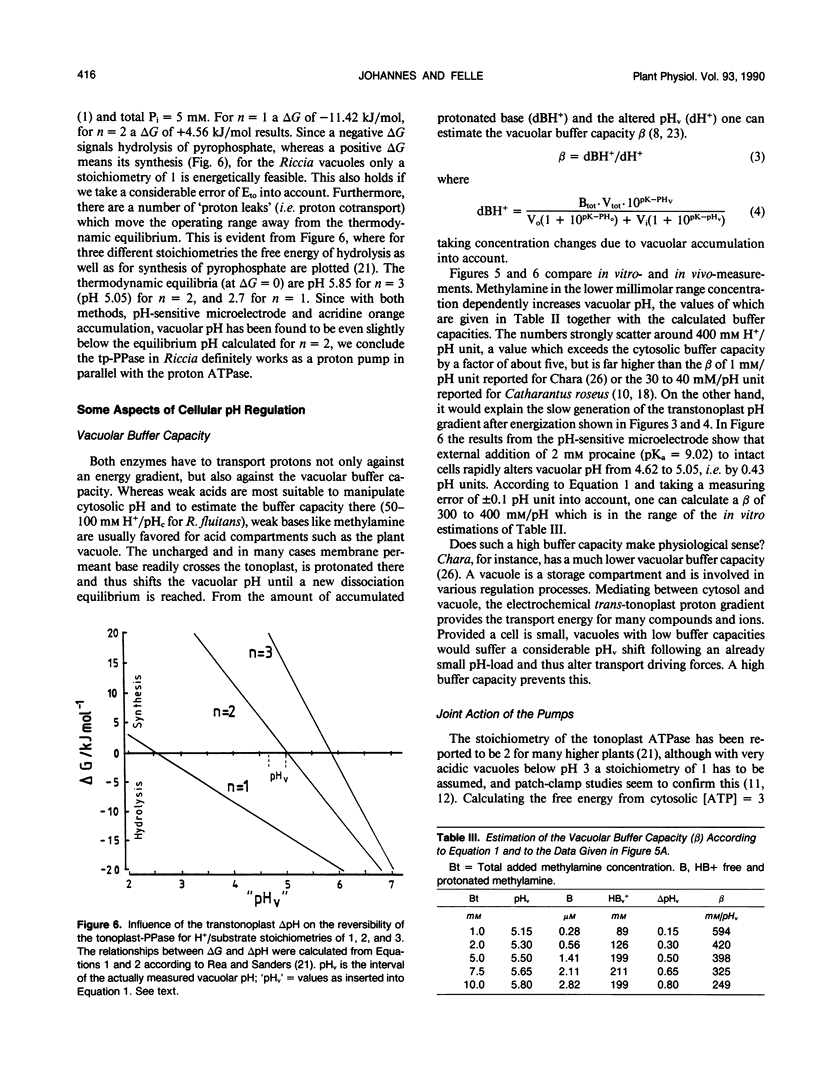
Using pH-sensitive microelectrodes (in vitro) and acridine orange photometry (in vivo), the actions of the two tonoplast phosphatases, the tp-ATPase and the tp-PPase, were investigated with respect to how effectively they could generate a transtonoplast pH-gradient. Under standard conditions the vacuoles of the aquatic liverwort Riccia fluitans have an in vivo pH of 4.7 to 5.0. In isolated vacuoles a maximal vacuolar pH (pHv) of 4.74 ± 0.1 is generated in the presence of 0.1 millimolar PPi, but only 4.93 ± 0.13 in the presence of 2.5 millimolar ATP. Both substrates added together approximate the value for PPi. Cl−-stimulates the H+-transport driven by the tp-ATPase, but has no effect on the tp-PPase. The transport activity of the tp-ATPase approximates saturation kinetics (K½ ≈ 0.5 millimolar), whereas transport by the tp-PPase yields an optimum around 0.1 millimolar PPi. The transtonoplast pH-gradient is dissipated slowly by weak bases, from which a vacuolar buffer capacity of roughly 300 to 400 millimolar/pHv unit has been estimated. From the free energy (−11.42 kilojoules per mole) for the hydrolysis of PPi under the given experimental conditions, we conclude that the PPase-stoichiometry (transported H+ per hydrolyzed substrate molecule) must be 1, and that in vivo this enzyme works as a H+-pump rather than as a pyrophosphate synthetase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentrup F. W., Gogarten-Boekels M., Hoffmann B., Gogarten J. P., Baumann C. ATP-dependent acidification and tonoplast hyperpolarization in isolated vacuoles from green suspension cells of Chenopodium rubrum L. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2431–2433. doi: 10.1073/pnas.83.8.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl A., Felle H., Bentrup F. W. Amine Transport in Riccia fluitans: Cytoplasmic and Vacuolar pH Recorded by a pH-Sensitive Microelectrode. Plant Physiol. 1984 Sep;76(1):75–78. doi: 10.1104/pp.76.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson A., Fichmann J., Spear D., Taiz L. Pyrophosphate-driven proton transport by microsomal membranes of corn coleoptiles. Plant Physiol. 1985 Sep;79(1):159–164. doi: 10.1104/pp.79.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guern J., Mathieu Y., Kurkdjian A., Manigault P., Manigault J., Gillet B., Beloeil J. C., Lallemand J. Y. Regulation of Vacuolar pH of Plant Cells: II. A P NMR Study of the Modifications of Vacuolar pH in Isolated Vacuoles Induced by Proton Pumping and Cation/H Exchanges. Plant Physiol. 1989 Jan;89(1):27–36. doi: 10.1104/pp.89.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R., Kurkdjian A. Characterization of an anion-permeable channel from sugar beet vacuoles: effect of inhibitors. EMBO J. 1988 Dec 1;7(12):3661–3666. doi: 10.1002/j.1460-2075.1988.tb03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu Y., Guern J., Kurkdjian A., Manigault P., Manigault J., Zielinska T., Gillet B., Beloeil J. C., Lallemand J. Y. Regulation of Vacuolar pH of Plant Cells: I. Isolation and Properties of Vacuoles Suitable for P NMR Studies. Plant Physiol. 1989 Jan;89(1):19–26. doi: 10.1104/pp.89.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea P. A., Poole R. J. Proton-Translocating Inorganic Pyrophosphatase in Red Beet (Beta vulgaris L.) Tonoplast Vesicles. Plant Physiol. 1985 Jan;77(1):46–52. doi: 10.1104/pp.77.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeille F., Bligny R., Douce R. Is the cytosolic pi concentration a limiting factor for plant cell respiration? Plant Physiol. 1984 Feb;74(2):355–359. doi: 10.1104/pp.74.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D., Slayman C. L. Control of intracellular pH. Predominant role of oxidative metabolism, not proton transport, in the eukaryotic microorganism Neurospora. J Gen Physiol. 1982 Sep;80(3):377–402. doi: 10.1085/jgp.80.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. A., Black C. C. Measurement of the pyrophosphate content of plant tissues. Plant Physiol. 1984 Jul;75(3):862–864. doi: 10.1104/pp.75.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshige K., Tazawa M. Measurement of the Cytoplasmic and Vacuolar Buffer Capacities in Chara corallina. Plant Physiol. 1989 Apr;89(4):1049–1052. doi: 10.1104/pp.89.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Leigh R. A., Kaestner K. H., Sze H. Electrogenic h-pumping pyrophosphatase in tonoplast vesicles of oat roots. Plant Physiol. 1986 Jun;81(2):497–502. doi: 10.1104/pp.81.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]


