Abstract
The evolution of medical knowledge about oral microbiota has increased awareness of its important role for the entire human body health. A wide range of microbial species colonizing the oral cavity interact both with each other and with their host through complex pathways. Usually, these interactions lead to a harmonious coexistence (i.e. eubiosis). However, several factors – including diet, poor oral hygiene, tobacco smoking, and certain medications, among others – can disrupt this weak homeostatic balance (i.e. dysbiosis) with potential implications on both oral (i.e. development of caries and periodontal disease) and systemic health. This article is thus aimed at providing an overview on the importance of oral microbiota in mediating several physiological and pathological conditions affecting human health. In this context, strategies based on oral hygiene and diet as well as the role of probiotics supplementation are discussed.
Keywords: Human microbiota, microbiome, oral dysbiosis, oral health, oral biofilm, dental caries, periodontitis, diet, probiotics
Impact Statement
This article extends the current knowledge on host–microbe interaction and cause-and-effect biomechanisms in the oral microbiota also in relation to other organs. The role of the oral microbiota as a mediator of several oral and systemic pathologic conditions as well as potential areas of intervention is discussed. The knowledge of both oral and other organ microbiota is continuously evolving with recent advances in the field. Despite future research being even needed, new promising therapeutic strategies targeted to the host microbiota are emerging with potential implications in the treatment of several oral and systemic pathologic conditions.
Introduction
The microbial communities in the oral cavity environment
The environment of the oral cavity presents both hard and mucous attachment areas (i.e. tongue, lips, cheeks, palate, and teeth); it is constantly moistened by saliva and in some areas by the gingival fluid providing nutrients to the microorganisms colonizing the mouth. The tooth surface supports the development and maturation of a complex biofilm.1,2 The properties of different areas change over the life course. The oral microbiome contains over 700 bacterial species as well as fungi, viruses, archaea, and protozoa.3,4 There are several factors influencing the microbiota composition such as age, dietary habits, alcohol, tabagism, social status, medications (i.e. antibiotics), toxic substances, pregnancy, and genetic predisposition. Sugars, fats, and vitamins are among the most impacting nutrients. In particular, fiber, medium-chain fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids have long been associated with both diversity and community structure of the oral microbiota.5,6 Sugar consumption has been associated with a marked presence of Streptococcus mutans and Fusobacterium nucleatum while it was seen that fats and vitamin C act as promoters of Fusobacteria. To maintain oral bacterial homeostasis, the evidence therefore suggests favoring fiber and dairy products.4,7,8 High alcohol consumption has been associated with an increase in Gram(+) bacteria including S. mutans and, in parallel, with an inhibition of the activity of Fusobacteria (due to an increase in acetaldehyde concentrations). Impurities, contaminants, fermentation, and distillation products can also cause changes in the oral environment that compromise the development of certain bacterial species.8,9 Some studies report that the oral microbiota of people in less well-off conditions is characterized by lower biodiversity with the presence of bacteria at higher concentrations such as Aggregatibacter segnis, Achromobacter xylosoxidans, and Neisseria cluster II. However, wealthy people have higher levels of Megasphaera micronuciformis, Veillonella atypical, Veillonella parvula, Rothia mucilaginosa, Prevotella histicola, Fusobacterium periodontium, Granulicatella adjacent, and Tannerella forsythia.8,10 Cigarette smoking has its own bacterial load which is transferred to the oral cavity such as Bacillus spp. and Clostridium spp. (new species from the outside). In addition, the alterations increase processes such as the acidification of saliva, the decrease in oxygen which leads to the development of anaerobic bacteria. Instead, bacteria such as Neisseria subflava and Corynebacterium are reduced in cigarette smokers.8,11 Antibiotics such as azithromycin, amoxicillin, clindamycin, and ciprofloxacin are antibiotics with the greatest impact on the microbiota leading to a decrease in Actinobacteria.8,12 Changes in bacterial functionality were also observed. During the first months of pregnancy, there is a greater expression of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans in the gingival sulcus while Candida is more present in the last trimester such as Capnocytophaga sputigena, Eubacterium saburreum, Fusobacterium nucleatum naviforme, Fusobacterium nucleatum polymorphum, Leptotrichia buccalis, Parvimonas micra, Prevotella intermedia, Prevotella melaninogenica, Staphylococcus aureus, Staphylococcus anginosus, Staphylococcus intermedius, Staphylococcus oralis, Staphylococcus mutans, Streptococcus sanguinis, Selenomonas noxia, and Veillonella parvula.8,13 The qualitative (biodiversity) and quantitative (number of species present) colonization of the microorganisms in humans appears to begin before birth. After birth, this colonization occurs in any area of the body (i.e. skin, oral cavity, nasopharynx, lung, intestine, system urogenital). New germs colonize the newborn’s body from the first minutes of its life and eventually form (such as the intestinal, oral, and others) within 10–13 years of life. Streptococcus spp. are the predominant bacteria in the oral microbiota during early childhood and the Streptococcus salivarius spp. carries out a protective action against potentially pathogenic species. In fact, S. salivarius K12 produces bacteriocins (salivaricin A2 and B) preventing various bacterial, fungal, viral, and autoimmune diseases in the oral cavity and in the upper airway tract.13,14 The newborn oral microbiota composition is affected by several factors like gestational age (i.e. full-term vs premature), type of feeding (i.e. breastfeeding vs formula feeding), mode of delivery (i.e. cesarean section vs vaginal delivery), and maternal nutritional status as well as exposure to antibiotics. 15 Through breast milk, several microorganisms are transferred to the newborn, promoting a better gastrointestinal function and a proper development of the immune system and microbiota promoting immunoregulatory cytokines, antibodies transfer, and antimicrobial agents selecting the microorganisms colonizing the gastrointestinal tract of the fetus.8,16 By the age of three years, microbiome composition that will endure for life is almost established. 15 The weaning phase, occurring at around four to six months and up to three years of the life of the newborn, is also important. A diet rich in antioxidants (fruits, vegetables, and other) helps the child keep a healthy microbiota. Finally, with the appearance of the first teeth, there are new surfaces for microbial colonization with a subsequent change after the replacement with the adult dentition.8,13,16 The oral colonization progress in line with the gut microbiota which constitutes the great biological organ communicating with the other microbiota via cross-talking axes such as oral/lung, gut/lung, gut/brain, gut /skin, gut/liver, and bladder/gut.17–19 The oral microbiota has a marked influence on the lung microbiota, thanks to the translocation of microorganisms, such as “bioaerosol” progression action, thus conveying them from the oral cavity to the lower airway’s tract. Therefore, the state of eubiosis of the oral microbiota is highly correlated to the pulmonary one, which in turn is linked to the intestinal one and consequently to all the axes of communication of the microbiota. In the gut microbiota, as occurs in the oral cavity, there are several bacteria like Bifidobacteria, Escherichia coli, Acidophilus influenzae, and Enterococci that help in preventing the proliferation of some pathogenic opportunistic bacteria.17,18,20
The oral microbiota in health and disease
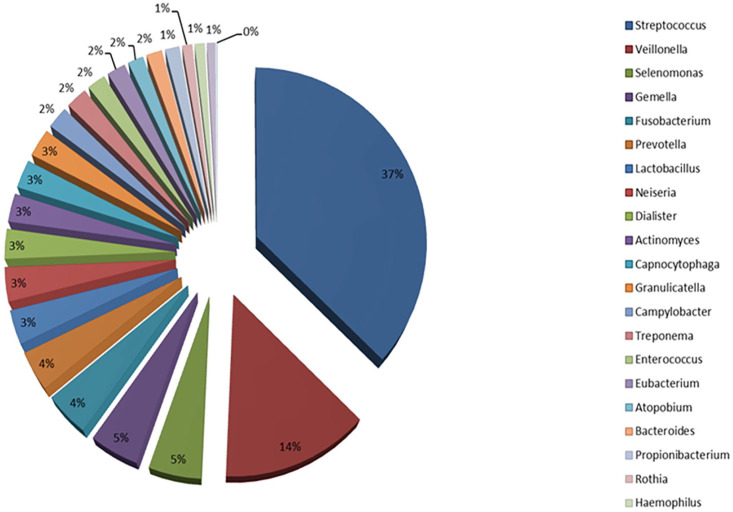
Today, one of the most important databases about oral human microbiota is the Human Oral Microbiome Database (eHOMD) (Figure 1).3,21 Typical types of Gram(+) bacteria in the oral cavity are the Streptococcus spp. Indeed, group A β-hemolytic Streptococcus (GABHS), is the most present of all. They can cause local infections such as tonsillopharyngitis, but also extra-oral infections such as acute otitis media in the ear, meningitis, pneumonia, and skin infections. Other bacterial species represented by S. mutans appears to promote dental caries by synthesizing extracellular and intracellular polysaccharides with acids production and influencing the formation of dental plaque. They are divided, according to antigens, into eight different serotypes.3,7,22 S. salivarius, which is an opportunistic pathogen found in large amounts in the saliva and that selectively colonizes the oral mucosa, produces extracellular polysaccharides in the presence of fructose and locally increases pH. The Streptococcus mitis is isolated from the oral mucosa and dental plaque. It is one of the first bacteria that colonize the oral cavity and is responsible for dental plaque. Under certain conditions, it causes bacteriemia after tooth extraction and endocarditis. Streptococcus vestibularis produce hydrogen peroxide and urease, from which ammonia is then produced, locally increasing the pH. S. anginosus is isolated from the mucous membranes, gingival sulcus, and dental plaque. It does not secrete polysaccharides and can play a protective role in caries but occasionally, S. anginosus can be pathogenic by mediating a range of diseases if transferred to various organs in the body.7,19,23 Staphylococcus spp. are present in the saliva, gingival fissure, in a minor percentage in dental plaque and mainly in immunocompromised subjects. About 50% of isolated Staphylococci spp. belong to the species S. aureus.7,23 Instead, Lactobacillus spp. presence is less than 1% and their proliferation is observed in the presence of caries. They could grow in a low-pH environment. From bacteria that can be opportunistic pathogens in the oral microbiota, Propionibacterium spp. and Corynebacterium spp. are noted.21,22,24 The Actinomyces are members of dental plaque flora. Some species are anaerobic and produce extracellular polysaccharides such as the Porphyromonas spp. They can bind to other types of bacteria and are involved in caries and gingivitis. Eubacteria, which are necessarily anaerobic, constitute 50% of the composition of the periodontal task and thus play an important role in the development of periodontitis.22,25 Unfortunately, Eubacteria cannot be easily cultivated and are difficult to isolate. Gram(−) bacteria are isolated from all surfaces of the oral cavity. They are involved in the formation of dental plaque and some species contribute to the development of caries. Porphyromonas spp. (particularly P. gingivalis) can mediate a range of oral diseases such as periodontitis and abscesses as well as other systemic diseases (i.e. endocarditis). Treponema denticola, another obligatory anaerobic Gram(–) pathogen, with a diffusive ability, may also be responsible for periodontitis.7,22,25,26 Among the microorganisms present in the oral microbiota, a small percentage is represented by the Archaea (few species and phylotypes). The Methanogen Archaea are today of greater clinical relevance since they have been detected in samples of periodontal plaque and in periodontitis. In particular, in patients with periodontitis, more than 30% of Archaea belong to the genus Methanobrevibacter (such as the Methanobrevibacter oralis, Methanobrevibacter cuticularis, Methanobrevibacter filiformi, Methanobrevibacter ruminantium, Methanobrevibacter arboriphilius), while other of the order Methanobacteriales belonging to Euryarchaeota such as those like Thermoplasm.7,8,27,28
Figure 1.
The main relative % phyla of oral microbiota stored in HOMD.
Source: HOMD, http://www.homd.org/.
Entamoeba gingivalis and Trichomonas tenax are the two protozoa species that have been identified in the oral cavity. They are mainly found in people with poor oral hygiene and in the presence of gingivitis. It has been found a high proportion of E. gingivalis compared to T. tenax in patients with gingival inflammation. However, they are generally considered non-pathogenic and probably their presence depends on nutrients as well as on the increase in the quantity of food and bacterial residues.21,22,29,30
The oral microbiota species of fungi that have been identified (i.e. Cladosporium, Saccharomyces, Aspergillus, Fusarium, and Cryptococcus, with the predominant Candida spp.) have a very small percentage of colonization. Finally, several viruses that can be found in the mouth are mainly related to diseases (i.e. mumps and rabies viruses which infect the salivary glands).22,26,31
The creation and development of the oral biofilm
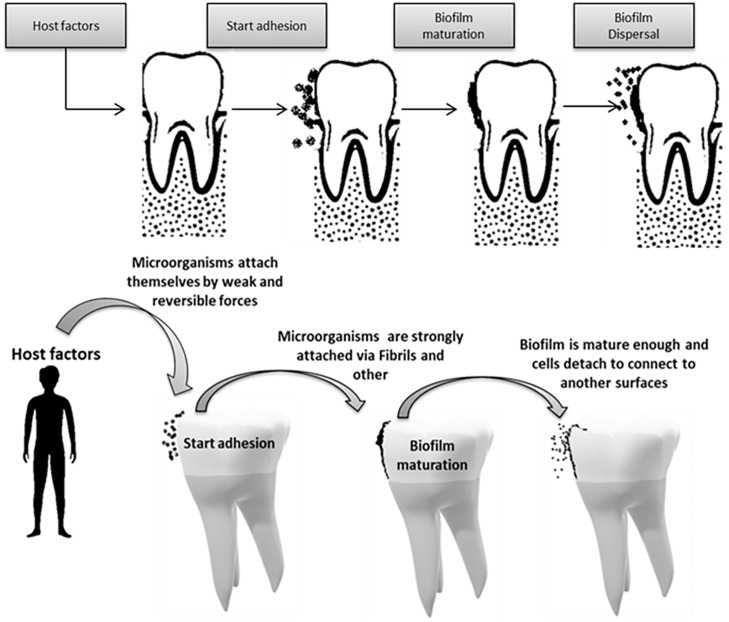
Biofilms are an organized community of microorganisms including bacteria, fungi, and protozoa, which are found in a network of polysaccharides and are attached to a living or inactive surface. Formerly, it was thought that bacteria were present in a homogeneous placental environment, but today it has been shown that bacteria seem to be biomechanical in their natural environment. 32 Their evolution is strongly influenced by Streptococcus spp., which are considered the main group of early settlers. Their initial adhesion determines the composition of subsequent settlers in the oral membrane and influences the health status of the host. The oral cavity has characteristics that make it, compared to other areas, unique as a substrate in the use of biomaterials as well as teeth, saliva, gingival fluid, and special surfaces of the mucosa.7,33 The normal microbial species of the mouth and of the dental plaque develop in the form of biomimetics. Immediately after birth, the sterile oral cavity is colonized by the so-called “first colonists,” which are Streptococci spp. (i.e. S. salivarius, S. mitis, and S. oralis) as well as the anaerobic strains P. melaninogenica, F. Nucleatum, and Veillonella spp.32–34 This first community, through its metabolic components, modifies the oral environment, creating favorable conditions for the settlement of other populations, called secondary settlers. As the teeth appear, new additional habitats are formed for germ colonization, as the teeth offer the only stable positions resulting in the constant accumulation of many bacteria.32,34,35 The biofilm has important properties. Microbial cells produce secretory polysaccharides that in turn cover and protect the biomimetic. Polysaccharides create a network of channels through which water flows, various nutrients and substances acting as biochemical signals. Bacterial biomembranes work together and interact with each other in different ways. Many of the interactions between different species are related to the competition for shared resources and the collaboration to create biofilms. Others are related to the configuration of communication with the host cells. It is important to note that microbial biomembranes exhibit different behaviors from free microbes of the same species.32,35 With the eruption of the primary teeth and then of the permanent ones, a special colonization is created on the enamel (i.e. the surface of the tooth) (Figure 2) creating a mature biomembrane of a mixed population of microbes from a dental surface (i.e. dental plaque). 32 When this biofilm is loosely attached to the teeth and easily removed with water, it is characterized by a white coating resulting harmful for the tooth. On contrary, when it shows a strong adhesion to the enamel and the organized colonies are removed only with strong mechanical pressure, it is characterized as dental microbial plaque.32,34,36 It is therefore a coating of microbes and products derived from them, rigidly fixed to the surface of the teeth in the form of a sort of biomembrane or biofilm. Depending on the location in which it develops, it is divided into coronal, supragingival, and subgingival. It develops in areas of the teeth that are not easily cleaned, such as the areas between the teeth, in the grooves, in the Howship lacunae and into gingival sulcus 32,34–37).
Figure 2.
The main stages of the biofilm formation process on the tooth surface.
Source: Charitos IA.
When oral hygiene does not take place properly, then the bacterial plaque will develop disproportionately. Immediately after cleaning the teeth, the surface is covered with organic salivary substances that form the acquired film or pellicle. This is what the original colonists, supported by hydrophobic bonds, and electrostatic forces of Van der Waals, initially attach to. Thus, the first bacteria bind permanently in such a way that they can no longer detach through adhesins polymers produced by bacteria and receptors present in the tissues in a measure not greater than 103/cm2. They are approached by saliva flow or, in some cases, by their own movement and begin to multiply secreting polysaccharides, while new bacteria are added and attached to the previous (secondary colonizers), with their number at about 105–106/cm2 in 5 min after cleaning the teeth. Bacteria interact with each other directly by contact through adhesives or indirectly by secretion of specific chemical molecules (i.e. biochemical communication).31,34,35 Interactions between dental plaque microorganisms are co-operative (i.e. nutrient production, co-occurrence in the catabolic decomposition of substances but also modification of existing environmental conditions) or competitive (i.e. competing for nutrients and available sites but also producing bactericides that affect other bacteria). The species of dental plaque microorganisms are quite different depending on their location on the teeth but also with individual variability. Most species are found in the supragingival plate (about 500) and less in the subgingival area (which is an extension under the gums at the root of the tooth). The number of microorganisms increases to a limit, where the plaque no longer increases, but reorganizes and changes constantly and it is then called a mature dental plaque of a mature biomembrane of a mixed population of dental surfaces.38–40
The saliva and teeth’s microbiota
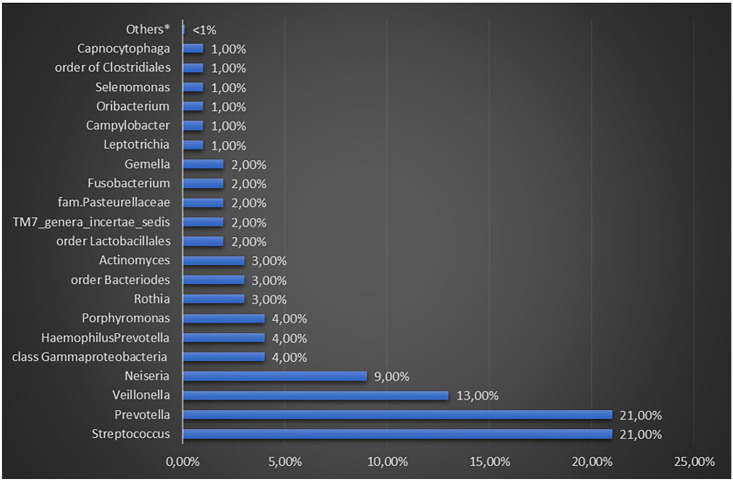
All microorganisms present in the saliva are derived from the oral tissue microcomposition by detaching themselves from their surface. However, the microbiota of saliva is mainly supplied by the biomembrane of the tongue (i.e. Prevotella and Streptococcus spp.) (Figure 3).22,41,42,43,44 Some genera of microorganisms (nearly 1%) present in the saliva can be considered transient or non-native. Given the presence of proteins and glycoproteins, it has been noted that they can also undermine both the qualitative and quantitative composition of the biomembranes.31,33 These microorganisms can promote microbial adhesion since salivary proteins overlap the surfaces of the mucous membranes and teeth. Teeth with their large surface area provide valid attachment support for the long-term development of the biomembrane.43–45 The sialic membrane covers the tooth enamel, while the roots are covered with a mixture of saliva and whey proteins. The microbial population of the corona has a slightly different composition from that of saliva with the predominance of Streptococcus spp. and Veillonella spp.31,33,45 The bacterial diversity of the dental plaque formed in the enamel (supragingival area above the gum line) is not the same as that below the gum line. It is important to note that the development of the bacterial community toward the roots therefore moves away from the salivary environment, since the film contains more serum and less saliva and becomes more anaerobic. 45
Figure 3.
Percentages of different genera recovered from the whole saliva of healthy adults.
Endogenous factors influencing oral microbiota eubiosis
Microorganisms need specific nutrients and environmental conditions to grow in the oral cavity, such as oxygen, proper temperature, and pH. Many microorganisms require oxygen to grow (aerobic), while others grow when it is absent from the environment (anaerobic). Although the oral cavity is rich in oxygen, it has also areas with anaerobic conditions, resulting in the survival of both aerobic and anaerobic microorganisms. Most organisms are optionally anaerobic or necessarily anaerobic,7,44,45 and most oxygen in the oral cavity is dissolved in the saliva and is readily available to microorganisms for their metabolic needs. The partial oxygen pressure (PO2) in the recently secreted saliva is about 65 mmHg, which immediately decreases to 35 mmHg only after 30–60 s. This decrease in oxygen levels continues to evolve over time as the plaque forms. The main factors responsible for the depletion of oxygen levels are the bacteria in the saliva and the use of oxygen to break down carbohydrates and nitrogenous substrates. 45 The progressive bacterial succession, the decrease of the PO2, and pH diminish the appearance of Gram(–) bacteria, oral infections, and halitosis. A study has shown that an oxidizing agent such as hydrogen peroxide can influence the biology of P. gingivalis. In addition, the growth of some members of the oral microcomposition can create oxidative and reductive conditions and therefore affect the ecology of these sites seriously affecting anaerobic bacteria such as P. gingivalis. 46 The normal oral cavity temperature is about 35–36°C, ideal for the development of mesophilic microorganisms. Temperature influences the growth rate of germs, as well as the solubility of gases in saliva, pH, adherence of microbes to dental surfaces, and ionic power. The temperature fluctuates between healthy people and in different areas of the mouth. In periodontitis, the temperature of the periodontal sac increases to about 39°C compared to wetlands. 46
Most microorganisms have a relatively narrow pH range to survive. Neutral pH is necessary for the growth of most bacteria in the oral cavity, while extreme pH values are not suitable for the growth of microorganisms. The main source of preservation of the pH of the oral cavity is saliva, which forms its value from about 6.75–7.25. 47 However, it has been found that different areas of the oral cavity have different pH. Oral areas that are less saturated with saliva have a more acidic or alkaline pH. After eating high-carbohydrate foods, the pH of the plaque drops to about 5.0, due to the formation of lactic acid with carbohydrate metabolism. However, this decline in pH usually returns slowly to initial levels over time. The pH reduction is lethal to most plaque bacteria. The pH of healthy gums is about 6.9 which increases to about 7.2–7.4 during pathologic conditions.47,48 This change in pH can lead to a change in gene expression in subcellular bacteria, which favors the development of pathogenic anaerobes such as P. gingivalis, which have an ideal pH of about 7.5. 46 P. gingivalis is an important colonizer of sublingual fissures and is an important pathogen in the onset and progression of severe forms of periodontal disease. Studies have shown that cotinine (a substance found in cigarette smokers) can interfere with P. gingivalis ability to bind and invade epithelial cells. However, further studies are needed to investigate whether oral epithelial cells may be more prone to colonization than P. gingivalis in smokers. 49
The metabolic products of some microorganisms are also food for other microorganisms. 8 Chewing act as a stimulator of the salivary flow. Saliva contains some dissolved nutrients such as glycoproteins, lipids, peptides, amino acids, minerals, vitamins, and gases which are used by oral bacteria as nutrient sources.32,50,51 Diet has a significant effect on the development of the bacterial and chemical composition of the biomembrane in the oral cavity. 8 Experimental animal studies have shown that diet has a slight influence on the early stages of biofilm development in the mouth. However, diet seems to later influence on a greater extent the proportions of the various bacterial species in the development of the biofilm. Dietary sugars provide immediately available substrates for oral microorganisms, most of which depend on carbohydrates to provide energy. 52 The metabolism of sucrose resulting from the diet of the species Streptococcus sanguis and S. mutans and the production of acids have a decisive effect on the composition of the microbiota and increase the mass of the dental plaque. Frequent consumption of carbohydrates also affects microbiota and other parts of the oral cavity. It has been found that people who consume high levels of high-frequency carbohydrates host more bacteria that grow better in an acidic environment, mainly Lactobacillus spp. and S. mutans, in their oral cavities.48,52 The acids produced by these bacteria are considered the main cause of dental caries. A metagenomics study based on rRNA was able to confirm a close relationship between the total glycemic load and the relative abundance of the Lactobacillaceae family.48,50,53
Main oral conditions affected by oral microbiota “imbalance”
Dental caries
Dental caries result from the destruction of the dental structure by acids produced through the fermentation of food carbohydrates operated by special bacteria that normally coexist in the mouth. The main causes of caries include microbial dental plaque, food sugars, and the acid environment in the mouth. Dental caries occur on any dental surface where dental plaque can grow for a long time. The creation of one of the dental plaques is an example of biofilm as above mentioned, and it happens through a natural process of interacting microorganisms.54,55 Some of these microorganisms can produce acids by metabolizing food carbohydrates with a pH reduction in the plaque within 1–3 min. When this process is repeated over time, the result is the demineralization of the dental surface. However, saliva can neutralize acids, increasing pH, and recovering lost ions from teeth in the so-called remineralization process. However, when the sum of these two processes results in the net loss of metal ions from the teeth, dental caries can evolve.56–58 The crown of the teeth can be further subdivided into separate areas and ecological sites, each of which is characterized by specific carries risks. Some sites host microbial communities that are acidic, produce acids, and can withstand an acidic environment (Figure 4). Therefore, the composition of the dental microbiota is not only influenced by teeth location in the mouth and its proximity to the flow of saliva from nearby ducts but also by the anatomy and physiology of the tooth surface and surrounding teeth.56,59
Figure 4.
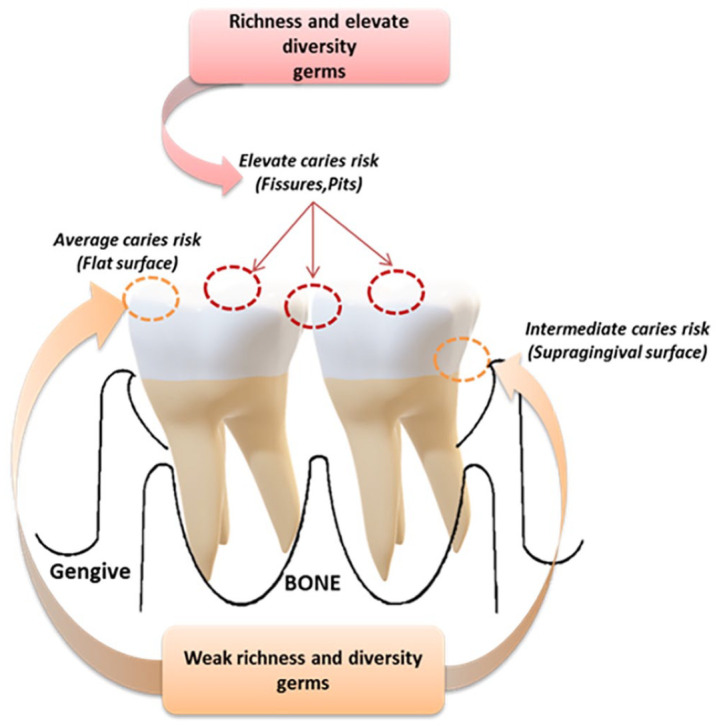
The diversity and richness of microbial communities are related to caries risk. Surfaces and positions with the highest variety and richness that are more sensitive to caries. When caries develops, the acidic environment reduces the variety and richness of local microbes.
Source: Charitos IA.
For many decades since the 1950s, S. mutans has been held the solely responsible for caries. More recent studies to determine the causative agent of the disease have produced vague results. It has been found that people with or without caries share about 50% of these microorganisms with higher amounts found in white spots lesions. 60 S. mutans seems to be associated only with the onset of caries (white spots) and appears to have the characteristics of a pathogen that is affected by dietary changes. In some patients with caries, Lactobacillus spp. and S. mutans are low or undetectable, suggesting that the onset and progression of lesions cannot be attributed solely to S. mutans.53,61 Indeed, S. mutans, Lactobacillus spp., Bifidobacterium spp., and Atopobium spp. have been detected in saliva only in people with decayed teeth. In fact, as previously mentioned, if the dental plaque is not removed (bad oral hygiene), the plaque bacteria release acids after the metabolisms of sugars presents, thus damaging the tooth enamel and facilitating the formation of caries.55,62 Overall, there is a change in the initial composition of the microbiota with the loss of microbial families leading to the development of caries. Organic acids derived from disaccharide hydrolysis such as sucrose and associated with caries also influence enzyme reactions. 55 An acidic environment in the mouth induces the production of protein enzymes and causes the modification of the microbial composition because they are selected aerobic and microorganisms that grow in an acidic environment. Caries reduce the diversity of microbes in relation to periodontal disease, probably due to the acidic environment, which limits microbial growth in organisms that do not grow in an acidic environment. Gingivitis and periodontitis are the main causes of tooth loss.55,63–65
Periodontal diseases
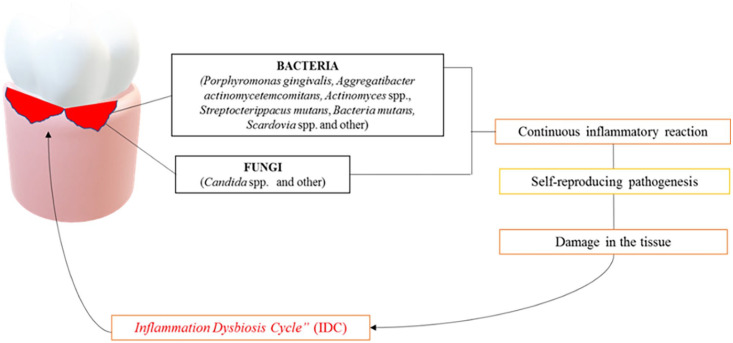
The main cause of periodontal disease is the development of dental plaque and tartar in the teeth due to poor oral hygiene. Gingivitis is perhaps the most common bacterial disease in humans. Dental plaque is constantly formed on tooth surfaces. Saliva glycoproteins form the salivary layer on the teeth to which the bacteria attach. The primary colonizer microorganisms can then interact with other bacteria and eventually contribute to the development of the biomembrane. The main colonizing microorganisms tend to be aerobic and optionally anaerobic Gram(+) such as Streptococcus and Actinomyces. Among the dominant species in the mature plaque, there are those anaerobic Gram(–) such as Fusobacterium and Treponema. 66 If oral hygiene is performed regularly, the dental plaque is kept immature and in a relatively small quantity. Otherwise, the accumulation of dental plaque can promote the growth and proliferation of several bacteria producing endotoxins and other enzymes that can enter the gums feeding an inflammatory response. The main clinical symptom of gingivitis is the bleeding of swollen gums. However, this condition is reversible if proper hygiene is restored. 67 Actinobacillus, P. gingivalis, Bacteroides forsythus, and Treponema denticola are among the main pathogenic species of anaerobic bacteria associated with the inflammatory response that leads to gingivitis. Periodontitis forms a periodontal pocket between the tooth and the gingiva that is colonized by anaerobic bacteria. The host contributes to the development of the lesion by producing proteases that damage the surrounding tissues. The teeth get mobility and eventually disappear. Periodontal disease is a polymicrobial infection that can be at least partially explained by the dysbiosis hypothesis.68–71 This hypothesis suggests that the low-frequency increase of the Gram(−) P. gingivalis can mediate the interaction with the host leading to inflammation and eventually to bone loss with the onset of periodontal disease.72–76 Furthermore, P. gingivalis limits host protection by facilitating the development of the entire microbial community through the modification of the nutrient medium. The modification of the analogous relationship between closely symbiotic microorganisms and pathogenic agents can lead to inflammation and subsequent bone destruction.77–79 However, despite the etiological composition of periodontal disease has not yet been revealed, beyond bacteria, various fungi can be a parameter responsible for the onset and progression of periodontal infection.80,81 In particular, at least 150 species of fungi belonging to the genera Ascomycota, Basidiomycota, Glomeromycota, and Chytridiomycota have been identified. In addition, a variety of unclassified organisms have also been reported in patients with periodontal diseases. To date, the discovery of the presence of Ascomycota in a measure of nearly 86.5% in both healthy and periodontal patients is of particular interest.82,83 Similarly, blood-borne viruses such as hepatitis viruses and HIV can enter the mouth and viruses related to upper respiratory infections can be clearly present in the mouth during the acute phase of infection.84–87 As above mentioned, oral dysbiosis can deflect an effective host immune response, leading to non-specific inflammatory reactions and tissue damage leading to an increase in the flow of gingival crevicular fluid (GCF) on small local hemorrhagic lesions (ulcers of the squamous epithelium). These in turn will lead to a deprivation of local oxygen in the area, favoring the development of anaerobic bacteria present in the gum cracks that in turn feed dysbiosis 55 and a sustained inflammatory reaction with further tissue damage, the so-called “inflammatory dysbiotic cycle” (IDC) (Figure 5). A recent study 88 evaluated the efficacy of a low-carbohydrate diet plan characterized by a particular attention to the daily intake of whole-grain products and fiber, omega-3 fatty acids, sources of vitamin C, vitamin D, and antioxidants in reducing inflammation and oxidative stress in treating periodontitis. The authors found that the application of this optimized diet was associated with a higher reduction in probing pocket depth (PPD) and full-mouth bleeding score (FMBS) after the treatment. 88
Figure 5.
The oral microbiota dysbiosis lead the overpopulation of certain bacterial strains but also fungi such as Candida albicans which in turn causes the local “Inflammation Dysbiosis Cycle condition.”
Source: Charitos IA.
Oral candidiasis
In healthy individuals, the Candida fungus has been found in mucous membranes such as the gastrointestinal tract, mouth, nose, reproductive organs, and skin. Oral candidiasis is a common occasional fungal infection of the oral mucosa caused by excessive growth of Candida spp. with the most common species Candida albicans.80,81 Factors contributing to its onset include salivary gland dysfunction, certain medications, artificial prosthetics, and high-carbohydrate diets. In the saliva, there are various antimicrobial substances such as lactoferrin, amylase, glycosylated proline-rich protein (PRP), lysozyme, and special antibodies against Candida.89,90 Additional dental components can contribute to Candida infection since they create a microenvironment leading to its development with anaerobic and low-pH Candida’s growth in saliva and its attachment to oral epithelial cells which is boosted by a carbohydrate-rich diet. Cigarette smoking, diabetes, cancer, and immunosuppression appear to be risk factors for oral candidiasis. The treatment of excessive growth of Candida does not seek to eliminate Candida from the individual, but to restore its correct and balanced ecological relationship between humans and microorganisms.80,91
Oral cancer
Oral cancer is a multifactorial disease. Recent studies reported that the presence of certain human papillomavirus (HPV) viruses (such as the HPV 16) and C. albicans can increase the tendency to malignant cell transformation. There is increasing evidence that HPV can act in combination with cigarette smoking to cause oral squamous cell carcinoma. 92 Recently, it has been reported a deterioration in the composition of overgrown fungi not only by C. albicans but also by the genera Hannaella and Gibberella in oral neoplasms that can be the causative agent of various diseases.80,92 Furthermore, around 40 species have been found in oral cancerous lesions including P. gingivalis, Porphyromonas endodontalis, Filifactor alocis, Dialister pneumosintes, and Tannerella forsythia. In this case, it is possible that the profile of the oral microbiota can function as a diagnostic biomarker in the prevention or diagnosis of those malignancies.93–101
Other pathological conditions
Dysbiosis of the oral cavity can have effects on other organs with or without systemic consequences. Hence, oral bacteria can play a role in several systemic diseases including lichen planus, leukoplakia, mucositis, cardiovascular disease, low respiratory airways tract, rheumatoid arthritis, lupus erythematosus, inflammatory bowel disease, cirrhosis, Alzheimer, polycystic ovary syndrome, obesity, diabetes, HIV infection, and extra-oral cancerous conditions (such as esophageal, colon, and pancreas cancers).102–108 Recent studies have demonstrated the presence of P. gingivalis in cancerous tissues of esophageal cancers. Also Peptostreptococcus, Fusobacterium, Peptococcus, Catonella, and Parvimonas micra have been associated with esophageal cancers (i.e. adenocarcinoma and squamous cell carcinoma).104,109
Some species colonizing the oral microbiota such as S. mutans, P. gingivalis, and Gemella haemolysans may also play an important role in the context of cardiovascular diseases. In fact, S. mutans, which is one of the main agents responsible for dental caries, has been shown to actively contribute to the development of atherosclerosis by altering the functionality of epithelial cells.110–112 P. gingivalis promotes the production of inflammatory cytokines (such as tumor necrosis factor α [TNF-α], interleukin (IL)-1β, IL-6, prostaglandin E2 [PGE2]) which in turn affect the cells of the atheromatous plaque with consequent aggravation of the condition of atherosclerosis. Campylobacter rectus, P. gingivalis, Porphyromonas endodontalis, and A. actinomycetemcomitans have been strongly associated with the development of coronary artery disease.113,114
The composition of the microbiota decreases along the path of the lower airways compared to that of the oral cavity. With the evolution of polymerase chain reaction (PCR) technology, the oral/lung microbiota axis has been investigated for its connection with some lung diseases through its dysbiosis.18,115 Oral microbiota dysbiosis may be thus linked to certain medical conditions of the lower airway tract such as pneumonia, cystic fibrosis, chronic obstructive pulmonary disease (COPD), and lung cancer.
Strategies to prevent oral dysbiosis
The mouthwashes’ role
Mouthwash plays an important role in preventing inflammation, infections, and the development of dental caries. Mouthwash with chlorhexidine gluconate has a wide antibacterial activity and therefore against Streptococcus spp., through its ability to persist for a long time after its use in both soft and hard tissues. Furthermore, chlorhexidine has a positive electrostatic charge, and therefore, it binds to negatively charged microbial cell membranes, which will lead to its destruction. It also blocks the activity of certain enzymes in microbes such as glycosyltransferases (GTFs) and phosphoenol pyruvate phosphatase. 116 Cetylpyridinium chloride is a quaternary ammonium compound that has antimicrobial activity has a smaller range of antimicrobial activity than chlorhexidine. It is absorbed into the oral surfaces and released at a faster rate than chlorhexidine. 116 Triclosan comes from 2,4,4’ trichloro-2-hydroxydiphenyl ether and zinc citrate has an extensive antimicrobial activity and is believed to inhibit cell membrane functions, especially in S. mutans. 117 Mouthwashes that contain phenol and four essential oils (thymol, eucalyptol, menthol, and methyl salicylate up to 26% alcohol) can modify the biofilm of plaque by removing most of the microorganisms that cause inflammation of the gums. Bacterial enzymes and endotoxins by Gram(–) bacteria have shown anti-inflammatory properties (i.e. through prostaglandin synthetase blockade). Delmopinol, derived from 2-morpholinoethanol, is effective in blocking both plaque and inflammation of the gums. 118 The property of fluoride against caries is due to three main mechanisms. First, fluorine ions act as active enzymatic substances blocking the metabolism of sugars by the microorganisms, resulting in a reduction or inhibition of organic acid production. A second mechanism involves the intracellular absorption which depends on the difference in pH between the inside of the bacteria and the external environment. Once inside the cell, fluorine ionizes releasing H + ions (HF ⇄ H ++ F−). The porous structure and the continuous calcification during tooth formation favor the greater incorporation of fluoride in the surface layer of the enamel. 119 Free fluoride ions in a solution can react with enamel and dentin crystals in different ways, depending on their concentration and composition of the solution. The presence of these forms reduces the solubility of the enamel at acidic pH. Finally, fluoride can increase the re-calcification rate by promoting the formation of apatite which is the only accelerator in the formation of apatite crystals. 120
Xylitol is a pentavalent alcohol that inhibits the growth of S. mutans. The mechanism consists of antagonizing glucose glycolysis by increasing the activity of amylase, carbonic anhydrase, and lactoperoxidase together with an increase in the regulatory capacity of saliva. There are several studies showing that the systemic use of xylitol reduces the number of S. mutans in saliva and the reduction in the incidence of caries.70,121,122,123 Sorbitol is not metabolized by most microorganisms in the oral cavity or slowly metabolized (such as the Lactobacillus spp.) relative to the time taken by the glucose components to ferment to acid. In fact, thanks this, it keeps the pH of saliva above the critical point (5.5 with the effect of its use on the prevention of caries). On the contrary, if there is long-term and frequent intake, it will lead to an increase in acid production (cause of the possible adaptation of microorganisms) and the pH falls below 5.5. A disadvantage of sorbitol is the incidence of gastrointestinal symptoms more evident than xylitol.122,124 Sanguinarine is an alkaloid derived from the plant Sanguinaria canadensis and is usually associated with zinc salts but its effect against S. mutans is lower than chlorhexidine due to its low bioavailability. Finally, povidone-iodine has a broad spectrum of activity against bacteria, fungi, protozoa, and viruses effective in reducing plaque and gingivitis and can be a useful complement to routine oral hygiene. In addition, it can reduce the severity of radiation mucositis.124,125
The action of the probiotics and prebiotics
Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host.” 126 However, prebiotics are “a substrate that is selectively utilized by host microorganisms conferring a health benefit.” 127 Prebiotics are mainly carbohydrate-based, although non-carbohydrate substances like polyphenols and polyunsaturated fatty acids converted to respective conjugated fatty acids have been reported. 127 Fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) currently represent the main prebiotic category. Prebiotics have several health effects including the reduction of inflammation and immune system stimulation as well as inhibition of pathogens and benefits for cardiometabolic and mental health.51,127 Prebiotics can be envisioned as nutrients for those probiotic bacteria. In fact, prebiotics stimulate probiotics bacteria like Lactobacilli and Bifidobacteria specifically, but not pathogens (i.e. certain Clostridia and Escherichia coli). 127 The potential role of probiotics on the oral cavity microbiota could be the same described for the gut. The use of probiotics to improve oral health has recently increased despite further studies are needed. In particular, Lactobacilli and Bifidobacteria can exert beneficial effects through three main mechanisms: (1) they can stimulate microbiota eubiosis, (2) they can lead to immunomodulation, and (3) they can mediate beneficial metabolic effects. Probiotics exert their function through several mechanisms including competition for adhesion sites, aggregation, competition for nutrient substrate and growth factors, production of antimicrobial compounds, increase of the efficiency of the immune system (i.e. improving the production of immunoglobulin A [IgA] and defensins), and inhibition of the synthesis of proinflammatory cytokines as well as reduction of the matrix metalloproteinases (MMPs) proenzymes production. Probiotics can thus inhibit the growth of pathogens and exert other effects related to the ecology of dental plaque, influencing the local and systemic immune response.128,129
Lactobacillus acidophilus showed promising effects in cases of periodontal disease, gingivitis, periodontitis, and pregnancy gingivitis. Regarding periodontal disease, Lactobacillus casei, Lactobacillus shirota, Lactobacillus reuteri have shown an effect in the reduction of gingival bleeding. Lactobacillus salivarius WB21 seems to reduce the depth of the gum pocket especially in cigarette smokers. L. casei and L. Shirota have been associated with a reduction of the activities of polymorphonuclear (PMN) elastase and MMP-3 in GCF. 130 The effects of Lactobacillus rhamnosus SP1 supplementation has been evaluated a randomized placebo-controlled trial as an adjunct to non-surgical periodontal therapy in patients with chronic periodontitis, but no differences were found among treated patients and placebo group. 131 Another study found that daily intake of heat-inactivated L. plantarum L-137 decreased the depth of periodontal pockets. 132 Alanzi et al. 133 in a randomized placebo-controlled clinical trial enrolling 108 healthy adolescents found that the administration of lozenge probiotics containing L. rhamnosus GG and Bifidobacterium lactis BB-12 improved gingival health and decreased the microbial count of harmful bacteria. Probiotics seem also to reduce sulfur gas production from the bacteria located mainly on the back of the tongue, with halitosis improvement. Probiotics can also help in balancing the pH in the mouth. Saliva is vital for oral health, the immune system, digestion and even in maintaining correct breathing. Probiotics contain a mixture of beneficial bacteria that help restore the natural and healthy production of saliva in the mouth with beneficial effects on xerostomia prevention.18,134,135 It was noted that the use of chewing gum containing probiotics with certain strains such as the L. reuteri ATCC PTA and 5289 ATCC 55730 can lead to a reduction of proinflammatory cytokines in GCF. Instead, the use of L. brevis resulted in a reduced action of MMP (collagenase) and other inflammatory markers in saliva. The use of some probiotic strains by lactating women appears to affect the composition of breast milk. As for commensal bacteria, there would always be several bacterial strains potentially useful in the prevention or treatment of oral diseases. 136 However, further investigation is needed to demonstrate the efficacy of orally administered bacteria on oral microbiota health in both adults and children. However, it is important to bear in mind that all the mechanisms studied are based on in vitro results and that clinical evidence is established in short pilot clinical studies. Several studies suggest that probiotics could reduce the number of S. mutans in saliva, toward a decrease in the number of Streptococcus spp. in saliva.137,138 Unfortunately, in the case of dental caries, the groups were relatively small with a short duration of follow-up. It seems that certain strains of Propionibacterium freudenreichii ssp. shermanii JS, L. rhamnosus LC705, and L. rhamnosus GG can help in reducing C. albicans concentrations. The S. salivarius K12 as a probiotic has shown beneficial effects in preventing tonsillitis from Streptococcus spp., viral infections and other inflammatory diseases. Finally, probiotics have been suggested to be effective in various infectious diseases such as viral including COVID-19 infection.139–142 As above mentioned, probiotics and prebiotics are frequently used in association for their symbiotic effects. 51 However, despite several studies have investigated the role of probiotics mainly on periodontal health, interventional studies on the effects of prebiotics on dental diseases are still lacking.51,143
Conclusions
Our knowledge of the world of microorganisms is constantly evolving. The recent advent of highly sensitive molecular techniques has greatly improved our knowledge of the synthesis of microbial communities present in various areas of the human body. With this important information, researchers are now ready to translate these results into a deeper understanding of the complex relationships between the oral microbiota and its host in health and disease. Although several interesting discoveries have already been made, there are still some important questions about microbial–host relationships to better understand the potential of novel therapeutic approaches in clinical practice. The microbiota of the oral cavity is the one that most influences the lung microbiota, and therefore, the eubiosis of the oral microbiota is strongly interrelated with the pulmonary one, which in turn exerts its influence on the gut microbiota. Oral microbiota dysbiosis, observed in many oral diseases (i.e. periodontal disease), can lead to the overpopulation of certain pathogen microorganisms (i.e. bacterial strains but also fungi such as C. albicans) leading to the so-called “IDC” condition. Finally, novel therapeutic strategies based on diet (i.e. limitation of simple sugars consumption) and probiotic supplementation could exert beneficial effects limiting the growth of those pathogen microorganisms implicated in some oral and systemic pathological conditions.
Footnotes
Authors’ Contributions: LS, PCP, DA, LB, and IAC contributed to conceptualizing and writing the manuscript. APC, MC, ST, FGG, and ADA edited and revised the manuscript. All authors approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Pier Carmine Passarelli  https://orcid.org/0000-0002-6416-8131
https://orcid.org/0000-0002-6416-8131
Domenico Azzolino  https://orcid.org/0000-0002-4910-7373
https://orcid.org/0000-0002-4910-7373
References
- 1. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007;449:804–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016;14:e1002533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Passarelli PC, Desantis V, De Angelis P, Piccirillo GB, Giovannini V, Papi P, Pompa G, D’Addona A. Prophylaxis with rFVIIa before third molar extraction in a patient with factor VII deficiency. J Biol Regul Homeost Agents 2020;34:679–82 [DOI] [PubMed] [Google Scholar]
- 4. Sultan AS, Kong EF, Rizk AM, Jabra-Rizk MA. The oral microbiome: a lesson in coexistence. PLoS Pathog 2018;14:e1006719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hansen TH, Kern T, Bak EG, Kashani A, Allin KH, Nielsen T, Hansen T, Pedersen O. Impact of a vegan diet on the human salivary microbiota. Sci Rep 2018;8:5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li X, Liu Y, Yang X, Li C, Song Z. The oral microbiota: community composition, influencing factors, pathogenesis, and interventions. Front Microbiol 2022;13:895537, https://www.frontiersin.org/articles/10.3389/fmicb.2022.895537 (accessed 3 February 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samaranayake L, Matsubara VH. Normal oral flora and the oral ecosystem. Dent Clin North Am 2017;61:199–215 [DOI] [PubMed] [Google Scholar]
- 8. Dagli N, Dagli R, Darwish S, Baroudi K. Oral microbial shift: factors affecting the microbiome and prevention of oral disease. J Contemp Dent Pract 2016;17:90–6 [DOI] [PubMed] [Google Scholar]
- 9. Fan X, Peters BA, Jacobs EJ, Gapstur SM, Purdue MP, Freedman ND, Alekseyenko AV, Wu J, Yang L, Pei Z, Hayes RB, Ahn J. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome 2018;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belstrøm D, Holmstrup P, Nielsen CH, Kirkby N, Twetman S, Heitmann BL, Klepac-Ceraj V, Paster BJ, Fiehn NE. Bacterial profiles of saliva in relation to diet, lifestyle factors, and socioeconomic status. J Oral Microbiol 2014;6. DOI: 10.3402/jom.v6.23609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu J, Peters BA, Dominianni C, Zhang Y, Pei Z, Yang L, Ma Y, Purdue MP, Jacobs EJ, Gapstur SM, Li H, Alekseyenko AV, Hayes RB, Ahn J. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J 2016;10:2435–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Almeida V, de SM, Azevedo J, Leal HF, Queiroz ATL, de Filho HP, da S, Reis JN. Bacterial diversity and prevalence of antibiotic resistance genes in the oral microbiome. PLoS ONE 2020;15:e0239664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saadaoui M, Singh P, Al Khodor S. Oral microbiome and pregnancy: a bidirectional relationship. J Reprod Immunol 2021;145:103293. [DOI] [PubMed] [Google Scholar]
- 14. Faa G, Gerosa C, Fanni D, Nemolato S, van Eyken P, Fanos V. Factors influencing the development of a personal tailored microbiota in the neonate, with particular emphasis on antibiotic therapy. J Matern Fetal Neonatal Med 2013;26:35–43 [DOI] [PubMed] [Google Scholar]
- 15. Agostoni C, Kim KS. Nutrition and the microbiome 2015. Pediatr Res 2015;77:113–4 [DOI] [PubMed] [Google Scholar]
- 16. Mändar R, Mikelsaar M. Transmission of mother’s microflora to the newborn at birth. Biol Neonate 1996;69:30–5 [DOI] [PubMed] [Google Scholar]
- 17. Santacroce L, Man A, Charitos IA, Haxhirexha K, Topi S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front Biosci Landmark Ed 2021;26:135–48 [DOI] [PubMed] [Google Scholar]
- 18. Mammen MJ, Scannapieco FA, Sethi S. Oral-lung microbiome interactions in lung diseases. Periodontol 2000 2020;83:234–41 [DOI] [PubMed] [Google Scholar]
- 19. Nobbs AH, Lamont RJ, Jenkinson HF. Streptococcus adherence and colonization. Microbiol Mol Biol Rev MMBR 2009;73:407–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Polimeno L, Barone M, Mosca A, Viggiani MT, Joukar F, Mansour-Ghanaei F, Mavaddati S, Daniele A, Debellis L, Bilancia M, Santacroce L, Di Leo A. Soy metabolism by gut microbiota from patients with precancerous intestinal lesions. Microorganisms 2020;8:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Human Oral Microbiome Database (HOMD), https://www.homd.org/ (accessed January 24, 2023).
- 22. Sudhakara P, Gupta A, Bhardwaj A, Wilson A. Oral dysbiotic communities and their implications in systemic diseases. Dent J 2018;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cong J, Zhang X. How human microbiome talks to health and disease. Eur J Clin Microbiol Infect Dis 2018;37:1595–601 [DOI] [PubMed] [Google Scholar]
- 24. Zhang Y, Wang X, Li H, Ni C, Du Z, Yan F. Human oral microbiota and its modulation for oral health. Biomed Pharmacother 2018;99:883–93 [DOI] [PubMed] [Google Scholar]
- 25. Siqueira JF, Rôças IN. The oral microbiota in health and disease: an overview of molecular findings. Methods Mol Biol Clifton NJ 2017;1537:127–38 [DOI] [PubMed] [Google Scholar]
- 26. Gao L, Xu T, Huang G, Jiang S, Gu Y, Chen F. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell 2018;9:488–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santacroce L, Sardaro N, Topi S, Pettini F, Bottalico L, Cantore S, Cascella G, Del Prete R, Dipalma G, Inchingolo F. The pivotal role of oral microbiota in health and disease. J Biol Regul Homeost Agents 2020;34:733–7 [DOI] [PubMed] [Google Scholar]
- 28. Poehlein A, Seedorf H. Draft genome sequences of methanobrevibacter curvatus DSM11111, methanobrevibacter cuticularis DSM11139, methanobrevibacter filiformis DSM11501, and methanobrevibacter oralis DSM7256. Genome Announc 2016;4:e00617–10016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rashidi Maybodi F, Haerian Ardakani A, Fattahi Bafghi A, Haerian Ardakani A, Zafarbakhsh A. The effect of nonsurgical periodontal therapy on Trichomonas tenax and Entamoeba gingivalis in patients with chronic periodontitis. J Dent Shiraz Iran 2016;17:171–6 [PMC free article] [PubMed] [Google Scholar]
- 30. Baker JL, Bor B, Agnello M, Shi W, He X. Ecology of the oral microbiome: beyond bacteria. Trends Microbiol 2017;25:362–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen T, Yu WH, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database J Biol Databases Curation 2010;2010:baq013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fux CA, Stoodley P, Hall-Stoodley L, Costerton JW. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Rev Anti Infect Ther 2003;1:667–83 [DOI] [PubMed] [Google Scholar]
- 33. Palmer RJ. Composition and development of oral bacterial communities. Periodontol 2000. 2014;64:20–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rendueles O, Ghigo JM. Multi-species biofilms: how to avoid unfriendly neighbors. FEMS Microbiol Rev 2012;36:972–89 [DOI] [PubMed] [Google Scholar]
- 35. Lemos JA, Abranches J, Koo H, Marquis RE, Burne RA. Protocols to study the physiology of oral biofilms. Methods Mol Biol Clifton NJ 2010;666:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mason MR, Chambers S, Dabdoub SM, Thikkurissy S, Kumar PS. Characterizing oral microbial communities across dentition states and colonization niches. Microbiome 2018;6:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li H, Xiao B, Zhang Y, Xiao S, Luo J, Huang W. Impact of maternal intrapartum antibiotics on the initial oral microbiome of neonates. Pediatr Neonatol 2019;60:654–61 [DOI] [PubMed] [Google Scholar]
- 38. Warinner C, Rodrigues JFM, Vyas R, Trachsel C, Shved N, Grossmann J, Radini A, Hancock Y, Tito RY, Fiddyment S, Speller C, Hendy J, Charlton S, Luder HU, Salazar-García DC, Eppler E, Seiler R, Hansen LH, Castruita JA, Barkow-Oesterreicher S, Teoh KY, Kelstrup CD, Olsen JV, Nanni P, Kawai T, Willerslev E, von Mering C, Lewis CM, Jr, Collins MJ, Gilbert MT, Rühli F, Cappellini E. Pathogens and host immunity in the ancient human oral cavity. Nat Genet 2014;46:336–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dzidic M, Collado MC, Abrahamsson T, Artacho A, Stensson M, Jenmalm MC, Mira A. Oral microbiome development during childhood: an ecological succession influenced by postnatal factors and associated with tooth decay. ISME J 2018;12:2292–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dewhirst FE. The oral microbiome: critical for understanding oral health and disease. J Calif Dent Assoc 2016;44:409–10 [PMC free article] [PubMed] [Google Scholar]
- 41. Wickström C, Herzberg MC, Beighton D, Svensäter G. Proteolytic degradation of human salivary MUC5B by dental biofilms. Microbiol Read Engl 2009;155:2866–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol 2003;30:644–54 [DOI] [PubMed] [Google Scholar]
- 43. Lis M, Bhatt S, Schoenly NE, Lee AY, Nislow C, Bobek LA. Chemical genomic screening of a Saccharomyces cerevisiae genomewide mutant collection reveals genes required for defense against four antimicrobial peptides derived from proteins found in human saliva. Antimicrob Agents Chemother 2013;57:840–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Keijser BJF, Zaura E, Huse SM, van der Vossen JMBM, Schuren FHJ, Montijn RC, ten Cate JM, Crielaard W. Pyrosequencing analysis of the oral microflora of healthy adults. J Dent Res 2008;87:1016–20 [DOI] [PubMed] [Google Scholar]
- 45. Faran Ali SM, Tanwir F. Oral microbial habitat a dynamic entity. J Oral Biol Craniofacial Res 2012;2:181–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Honda K. Porphyromonas gingivalis sinks teeth into the oral microbiota and periodontal disease. Cell Host Microbe 2011;10:423–5 [DOI] [PubMed] [Google Scholar]
- 47. Marsh PD. Dental plaque as a biofilm: the significance of pH in health and caries. Compend Contin Educ Dent 2009;30 76–8, 80, 83–7; quiz 88, 90 [PubMed] [Google Scholar]
- 48. Wade WG. The oral microbiome in health and disease. Pharmacol Res 2013;69:137–43 [DOI] [PubMed] [Google Scholar]
- 49. Cogo K, Calvi BM, Mariano FS, Franco GCN, Gonçalves RB, Groppo FC. The effects of nicotine and cotinine on Porphyromonas gingivalis colonisation of epithelial cells. Arch Oral Biol 2009;54:1061–7 [DOI] [PubMed] [Google Scholar]
- 50. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 2005;43:5721–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Santonocito S, Giudice A, Polizzi A, Troiano G, Merlo EM, Sclafani R, Grosso G, Isola G. A cross-talk between diet and the oral microbiome: balance of nutrition on inflammation and immune system’s response during periodontitis. Nutrients 2022;14:2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bowden GH, Li YH. Nutritional influences on biofilm development. Adv Dent Res 1997;11:81–99 [DOI] [PubMed] [Google Scholar]
- 53. Gross EL, Leys EJ, Gasparovich SR, Firestone ND, Schwartzbaum JA, Janies DA, Asnani K, Griffen AL. Bacterial 16S sequence analysis of severe caries in young permanent teeth. J Clin Microbiol 2010;48:4121–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krishnan K, Chen T, Paster BJ. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis 2017;23:276–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dipalma G, Inchingolo AD, Inchingolo F, Charitos IA, Di Cosola M, Cazzolla AP. Focus on the cariogenic process: microbial and biochemical interactions with teeth and oral environment. J Biol Regul Homeost Agents 2021;35:429–440 [DOI] [PubMed] [Google Scholar]
- 56. Barbería E, Maroto M, Arenas M, Silva CC. A clinical study of caries diagnosis with a laser fluorescence system. J Am Dent Assoc 1939 2008;139:572–9 [DOI] [PubMed] [Google Scholar]
- 57. Belibasakis GN, Bostanci N, Marsh PD, Zaura E. Applications of the oral microbiome in personalized dentistry. Arch Oral Biol 2019;104:7–12 [DOI] [PubMed] [Google Scholar]
- 58. Di Domenico M, Ballini A, Boccellino M, Scacco S, Lovero R, Charitos IA, Santacroce L. The intestinal microbiota may be a potential theranostic tool for personalized medicine. J Pers Med 2022;12:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Struzycka I. The oral microbiome in dental caries. Pol J Microbiol 2014;63:127–35 [PubMed] [Google Scholar]
- 60. Peterson SN, Snesrud E, Liu J, Ong AC, Kilian M, Schork NJ, Bretz W. The dental plaque microbiome in health and disease. PLoS ONE 2013;8:e58487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Belda-Ferre P, Alcaraz LD, Cabrera-Rubio R, Romero H, Simón-Soro A, Pignatelli M, Mira A. The oral metagenome in health and disease. ISME J 2012;6:46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Loesche W. Dental caries and periodontitis: contrasting two infections that have medical implications. Infect Dis Clin North Am 2007;21:471–502 [DOI] [PubMed] [Google Scholar]
- 63. Passarelli PC, Lajolo C, Pasquantonio G, D’Amato G, Docimo R, Verdugo F, D’Addona A. Influence of mandibular third molar surgical extraction on the periodontal status of adjacent second molars. J Periodontol 2019;90:847–55 [DOI] [PubMed] [Google Scholar]
- 64. Sun F, Ahmed A, Wang L, Dong M, Niu W. Comparison of oral microbiota in orthodontic patients and healthy individuals. Microb Pathog 2018;123:473–7 [DOI] [PubMed] [Google Scholar]
- 65. Ballini A, Cantore S, Dedola A, Santacroce L, Laino L, Cicciù M, Mastrangelo F. IL-1 haplotype analysis in periodontal disease. J Biol Regul Homeost Agents 2018;32:433–7 [PubMed] [Google Scholar]
- 66. Zijnge V, van Leeuwen MBM, Degener JE, Abbas F, Thurnheer T, Gmür R, Harmsen HJ. Oral biofilm architecture on natural teeth. PLoS ONE 2010;5:e9321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cafiero C, Grippaudo C, Dell’Aquila M, Cimmino P, D’Addona A, De Angelis P, Ottaiano MP, Costagliola D, Benincasa G, Micera A, Santacroce L, Palmirotta R. Association between Vitamin D receptor gene polymorphisms and periodontal bacteria: a clinical pilot study. Biomolecules 2022;12:833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 2011;10:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Passarelli PC, De Angelis P, Pasquantonio G, Manicone PF, Verdugo F, D’Addona A. Management of single uncomplicated dental extractions and postoperative bleeding evaluation in patients with factor V deficiency: a local antihemorrhagic approach. J Oral Maxillofac Surg 2018;76:2280–3 [DOI] [PubMed] [Google Scholar]
- 70. Ballini A, Cantore S, Signorini L, Saini R, Scacco S, Gnoni A, Inchingolo AD, De Vito D, Santacroce L, Inchingolo F, Dipalma G. Efficacy of sea salt-based mouthwash and xylitol in improving oral hygiene among adolescent population: a pilot study. Int J Environ Res Public Health 2020;18:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ballini A, Santacroce L, Cantore S, Saini R, Mastrangelo F, Desiate A, Scacco S. Orthodontic forces modulate insulin-like growth factor binding protein-5 changes in gingival crevicular fluid. J Biol Regul Homeost Agents 2016;30:1235–40 [PubMed] [Google Scholar]
- 72. Colombo APV, Bennet S, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, Van Dyke TE, Dewhirst FE, Paster BJ. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: comparison between good responders and “refractory” subjects by the Human Oral Microbe Identification Microarray (HOMIM). J Periodontol 2012;83:1279–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Papi P, Di Murro B, Tromba M, Passarelli PC, D’Addona A, Pompa G. The use of a non-absorbable membrane as an occlusive barrier for alveolar ridge preservation: a one year follow-up prospective cohort study. Antibiotics 2020;9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kumar PS. From focal sepsis to periodontal medicine: a century of exploring the role of the oral microbiome in systemic disease. J Physiol 2017;595:465–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Passarelli PC, Saccomanno S, De Angelis P, Romeo A, Piccirillo GB, Desantis V, Grippaudo C, D’Addona A. Study of cellular toxicity in vitro of two resins for orthodontic use. Eur Rev Med Pharmacol Sci 2020;24:930–4 [DOI] [PubMed] [Google Scholar]
- 76. Jia G, Zhi A, Lai PFH, Wang G, Xia Y, Xiong Z, Zhang H, Che N, Ai L. The oral microbiota – a mechanistic role for systemic diseases. Br Dent J 2018;224:447–55 [DOI] [PubMed] [Google Scholar]
- 77. Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol 2012;27:409–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Passarelli PC, Pasquantonio G, D’Addona A. Management of surgical third lower molar extraction and postoperative progress in patients with factor VII deficiency: a clinical protocol and focus on this rare pathologic entity. J Oral Maxillofac Surg 2017;75:2070.e1–4 [DOI] [PubMed] [Google Scholar]
- 79. van der Meulen TA, Harmsen H, Bootsma H, Spijkervet F, Kroese F, Vissink A. The microbiome-systemic diseases connection. Oral Dis 2016;22:719–34 [DOI] [PubMed] [Google Scholar]
- 80. Giudice G, Cutrignelli DA, Sportelli P, Limongelli L, Tempesta A, Gioia GD, Santacroce L, Maiorano E, Favia G. Rhinocerebral mucormycosis with orosinusal involvement: diagnostic and surgical treatment guidelines. Endocr Metab Immune Disord Drug Targets 2016;16:264–9 [DOI] [PubMed] [Google Scholar]
- 81. Sztukowska MN, Dutton LC, Delaney C, Ramsdale M, Ramage G, Jenkinson HF, Nobbs AH, Lamont RJ. Community development between Porphyromonas gingivalis and Candida albicans mediated by InlJ and Als3. mBio 2018;9:e00202–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Santacroce L, Muzio EL, Bottalico L, Spirito F, Charitos IA, Passarelli PC, Jirillo E. Subversion of the oral microbiota and induction of immune-mediated systemic inflammation with special reference to periodontitis: current knowledge and perspectives. Endocr Metab Immune Disord Drug Targets 2022;23:470–84 [DOI] [PubMed] [Google Scholar]
- 83. Topi S, Bottalico L, Charitos IA, Colella M, Di Domenico M, Palmirotta R, Santacroce L. Biomolecular mechanisms of autoimmune diseases and their relationship with the resident microbiota: friend or foe? Pathophysiology 2022;29:507–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Passarelli PC, Pasquantonio G, Manicone PF, Cerroni L, Condo’ R, Mancini M, D’Addona A. Orofacial signs and dental abnormalities in patients with Mulvihill-Smith syndrome: a literature review on this rare progeroid pathology. Medicine (Baltimore) 2018;97:e0656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Saccomanno S, Passarelli PC, Oliva B, Grippaudo C. Comparison between two radiological methods for assessment of tooth root resorption: an in vitro study. Biomed Res Int 2018;2018:5152172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Santacroce L, Carlaio RG, Bottalico L. Does it make sense that diabetes is reciprocally associated with periodontal disease? Endocr Metab Immune Disord Drug Targets 2010;10:57–70 [DOI] [PubMed] [Google Scholar]
- 87. Vanhoecke B, De Ryck T, Stringer A, Van de Wiele T, Keefe D. Microbiota and their role in the pathogenesis of oral mucositis. Oral Dis 2015;21:17–30 [DOI] [PubMed] [Google Scholar]
- 88. De Angelis P, Gasparini G, Manicone PF, Passarelli PC, Azzolino D, Rella E, De Rosa G, Papi P, Pompa G, De Angelis S, Grassi R, D’Addona A. The effect of an optimized diet as an adjunct to non-surgical periodontal therapy in subjects with periodontitis: a prospective study. Healthcare 2022;10:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Akpan A, Morgan R. Oral candidiasis. Postgrad Med J 2002;78:455–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Man A, Ciurea CN, Pasaroiu D, Savin AI, Toma F, Sular F, Santacroce L, Mare A. New perspectives on the nutritional factors influencing growth rate of Candida albicans in diabetics. An in vitro study. Mem Inst Oswaldo Cruz 2017;112:587–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Monea A, Santacroce L, Marrelli M, Man A. Oral candidiasis and inflammatory response: a potential synergic contribution to the onset of type-2 diabetes mellitus. Australas Med J 2017;10:550–6 [Google Scholar]
- 92. Santacroce L, Di Cosola M, Bottalico L, Topi S, Charitos IA, Ballini A, Inchingolo F, Cazzolla AP, Dipalma G. Focus on HPV infection and the molecular mechanisms of oral carcinogenesis. Viruses 2021;13:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bollero P, Passarelli PC, D’Addona A, Pasquantonio G, Mancini M, Condò R, Cerroni L. Oral management of adult patients undergoing hematopoietic stem cell transplantation. Eur Rev Med Pharmacol Sci 2018;22:876–87 [DOI] [PubMed] [Google Scholar]
- 94. Ayuningtyas NF, Mahdani FY, Pasaribu TAS, Chalim M, Ayna VKP, Santosh ABR, Santacroce L, Surboyo MDC. Role of Candida albicans in oral carcinogenesis. Pathophysiology 2022;29:650–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Le Bars P, Matamoros S, Montassier E, Le Vacon F, Potel G, Soueidan A, Jordana F, de La Cochetière MF. The oral cavity microbiota: between health, oral disease, and cancers of the aerodigestive tract. Can J Microbiol 2017;63:475–92 [DOI] [PubMed] [Google Scholar]
- 96. Inchingolo F, Santacroce L, Ballini A, Topi S, Dipalma G, Haxhirexha K, Bottalico L, Charitos IA. Oral cancer: a historical review. Int J Environ Res Public Health 2020;17:3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Saini R, Cantore S, Saini SR, Mastrangelo F, Ballini A, Santacroce L. Efficacy of fluorescence technology vs conventional oral examination for the early detection of oral pre-malignant lesions. A clinical comparative study. Endocr Metab Immune Disord Drug Targets 2019;19:852–8 [DOI] [PubMed] [Google Scholar]
- 98. Schmidt BL, Kuczynski J, Bhattacharya A, Huey B, Corby PM, Queiroz ELS, Nightingale K, Kerr AR, DeLacure MD, Veeramachaneni R, Olshen AB, Albertson DG. Changes in abundance of oral microbiota associated with oral cancer. PLoS ONE 2014;9:e98741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yang SF, Huang HD, Fan WL, Jong YJ, Chen MK, Huang CN, Chuang CY, Kuo YL, Chung WH, Su SC. Compositional and functional variations of oral microbiota associated with the mutational changes in oral cancer. Oral Oncol 2018;77:1–8 [DOI] [PubMed] [Google Scholar]
- 100. Gholizadeh P, Eslami H, Yousefi M, Asgharzadeh M, Aghazadeh M, Kafil HS. Role of oral microbiome on oral cancers, a review. Biomed Pharmacother 2016;84:552–8 [DOI] [PubMed] [Google Scholar]
- 101. Lim Y, Totsika M, Morrison M, Punyadeera C. Oral microbiome: a new biomarker reservoir for oral and oropharyngeal cancers. Theranostics 2017;7:4313–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. La Rosa GRM, Gattuso G, Pedullà E, Rapisarda E, Nicolosi D, Salmeri M. Association of oral dysbiosis with oral cancer development. Oncol Lett 2020;19:3045–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chen J, Domingue JC, Sears CL. Microbiota dysbiosis in select human cancers: evidence of association and causality. Semin Immunol 2017;32:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Graves DT, Corrêa JD, Silva TA. The oral microbiota is modified by systemic diseases. J Dent Res 2019;98:148–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Flemer B, Warren RD, Barrett MP, Cisek K, Das A, Jeffery IB, Hurley E, O’Riordain M, Shanahan F, O’Toole PW. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2018;67:1454–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ballini A, Scacco S, Boccellino M, Santacroce L, Arrigoni R. Microbiota and obesity: where are we now? Biology (Basel) 2020;9:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Komiya Y, Shimomura Y, Higurashi T, Sugi Y, Arimoto J, Umezawa S, Uchiyama S, Matsumoto M, Nakajima A. Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut 2019;68:1335–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Olson SH, Satagopan J, Xu Y, Ling L, Leong S, Orlow I, Saldia A, Li P, Nunes P, Madonia V, Allen PJ, O’Reilly E, Pamer E, Kurtz RC. The oral microbiota in patients with pancreatic cancer, patients with IPMNs, and controls: a pilot study. Cancer Causes Control 2017;28:959–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gao S, Li S, Ma Z, Liang S, Shan T, Zhang M, Zhu X, Zhang P, Liu G, Zhou F, Yuan X, Jia R, Potempa J, Scott DA, Lamont RJ, Wang H, Feng X. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect Agent Cancer 2016;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Schirinzi A, Cazzolla AP, Lovero R, Lo Muzio L, Testa NF, Ciavarella D, Palmieri G, Pozzessere P, Procacci V, Di Serio F, Santacroce L. New insights in laboratory testing for COVID-19 patients: looking for the role and predictive value of human epididymis secretory protein 4 (HE4) and the innate immunity of the oral cavity and respiratory tract. Microorganisms 2020;8:1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wang Y, Xue J, Zhou X, You M, Du Q, Yang X, He J, Zou J, Cheng L, Li M, Li Y, Zhu Y, Li J, Shi W, Xu X. Oral microbiota distinguishes acute lymphoblastic leukemia pediatric hosts from healthy populations. PLoS ONE 2014;9:e102116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lazar V, Ditu LM, Pircalabioru GG, Gheorghe I, Curutiu C, Holban AM, Picu A, Petcu L, Chifiriuc MC. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol 2018;9:1830, https://www.frontiersin.org/articles/10.3389/fimmu.2018.01830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Krzyściak W, Jurczak A, Kościelniak D, Bystrowska B, Skalniak A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis 2014;33:499–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Poole S, Singhrao SK, Chukkapalli S, Rivera M, Velsko I, Kesavalu L, Crean S. Active invasion of Porphyromonas gingivalis and infection-induced complement activation in ApoE−/− mice brains. J Alzheimers Dis JAD 2015;43:67–80 [DOI] [PubMed] [Google Scholar]
- 115. Santacroce L, Charitos IA, Ballini A, Inchingolo F, Luperto P, De Nitto E, Topi S. The human respiratory system and its microbiome at a glimpse. Biology 2020;9:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Basrani B, Lemonie C. Chlorhexidine gluconate. Aust Endod J 2005;31:48–52 [DOI] [PubMed] [Google Scholar]
- 117. Abello R, Buitrago C, Prate CM, DeVizio W, Bakar SK. Effect of a mouthrinse containing triclosan and a copolymer on plaque formation in the absence of oral hygiene. Am J Dent 1990;3:S57–61 [PubMed] [Google Scholar]
- 118. Raja M, Saha S, Vamsi K, Reddy Shafaat M, Kumari M. Mouthwashes – an overview of current knowledge. Int J Oral Health Res Rev 2013;1:24–8 [Google Scholar]
- 119. Jenkins GN. Review of fluoride research since 1959. Arch Oral Biol 1999;44:985–92 [DOI] [PubMed] [Google Scholar]
- 120. Aoba T, Shimazu Y, Taya Y, Soeno Y, Sato K, Miake Y. Fluoride and apatite formation in vivo and in vitro. J Electron Microsc (Tokyo) 2003;52:615–25 [DOI] [PubMed] [Google Scholar]
- 121. Machiulskiene V, Nyvad B, Baelum V. Caries preventive effect of sugar-substituted chewing gum. Community Dent Oral Epidemiol 2001;29:278–88 [DOI] [PubMed] [Google Scholar]
- 122. Söderling E, Isokangas P, Tenovuo J, Mustakallio S, Mäkinen KK. Long-term xylitol consumption and mutans Streptococci in plaque and saliva. Caries Res 1991;25:153–7 [DOI] [PubMed] [Google Scholar]
- 123. Corsalini M, Montagnani M, Charitos IA, Bottalico L, Barile G, Santacroce L. Non-surgical therapy and oral microbiota features in peri-implant complications: a brief narrative review. Healthcare (Basel) 2023;11:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Petersen PE, Razanamihaja N. Carbamide-containing polyol chewing gum and prevention of dental caries in schoolchildren in Madagascar. Int Dent J 1999;49:226–30 [DOI] [PubMed] [Google Scholar]
- 125. Brecx M, Netuschil L, Reichert B, Schreil G. Efficacy of listerine, meridol and chlorhexidine mouthrinses on plaque, gingivitis and plaque bacteria vitality. J Clin Periodontol 1990;17:292–7 [DOI] [PubMed] [Google Scholar]
- 126. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. The International Scientific Association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–14 [DOI] [PubMed] [Google Scholar]
- 127. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 2017;14:491–502 [DOI] [PubMed] [Google Scholar]
- 128. Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Ther Adv Gastroenterol 2013;6:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Santacroce L, Charitos IA, Bottalico L. A successful history: probiotics and their potential as antimicrobials. Expert Rev Anti Infect Ther 2019;17:635–45 [DOI] [PubMed] [Google Scholar]
- 130. Chugh P, Dutt R, Sharma A, Bhagat N, Dhar MS. A critical appraisal of the effects of probiotics on oral health. J Funct Foods 2020;70:103985 [Google Scholar]
- 131. Morales A, Carvajal P, Silva N, Hernandez M, Godoy C, Rodriguez G, Garcia-Sesnich J, Hoare A, Diaz PI, Gamonal J. Clinical effects of Lactobacillus rhamnosus in non-surgical treatment of chronic periodontitis: a randomized placebo-controlled trial with 1-year follow-up. J Periodontol 2016;87:944–52 [DOI] [PubMed] [Google Scholar]
- 132. Iwasaki K, Maeda K, Hidaka K, Nemoto K, Hirose Y, Deguchi S. Daily intake of heat-killed Lactobacillus plantarum l-137 decreases the probing depth in patients undergoing supportive periodontal therapy. Oral Health Prev Dent 2016;14:207–14 [DOI] [PubMed] [Google Scholar]
- 133. Alanzi A, Honkala S, Honkala E, Varghese A, Tolvanen M, Söderling E. Effect of Lactobacillus rhamnosus and Bifidobacterium lactis on gingival health, dental plaque, and periodontopathogens in adolescents: a randomised placebo-controlled clinical trial. Benef Microbes 2018;9:593–602 [DOI] [PubMed] [Google Scholar]
- 134. Cantore S, Crincoli V, Boccaccio A, Uva AE, Fiorentino M, Monno G, Bollero P, Derla C, Fabiano F, Ballini A, Santacroce L. Recent advances in endocrine, metabolic and immune disorders: mesenchymal stem cells (MSCs) and engineered scaffolds. Endocr Metab Immune Disord Drug Targets 2018;18:466–9 [DOI] [PubMed] [Google Scholar]
- 135. Passarelli PC, Pagnoni S, Piccirillo GB, Desantis V, Benegiamo M, Liguori A, Papa R, Papi P, Pompa G, D’Addona A. Reasons for tooth extractions and related risk factors in adult patients: a cohort study. Int J Environ Res Public Health 2020;17:2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Twetman S, Derawi B, Keller M, Ekstrand K, Yucel-Lindberg T, Stecksen-Blicks C. Short-term effect of chewing gums containing probiotic Lactobacillus reuteri on the levels of inflammatory mediators in gingival crevicular fluid. Acta Odontol Scand 2009;67:19–24 [DOI] [PubMed] [Google Scholar]
- 137. Isacco CG, Ballini A, De Vito D, Nguyen KCD, Cantore S, Bottalico L, Quagliuolo L, Boccellino M, Di Domenico M, Santacroce L, Arrigoni R, Dipalma G, Inchingolo F. Rebalancing the oral microbiota as an efficient tool in endocrine, metabolic and immune disorders. Endocr Metab Immune Disord Drug Targets 2021;21:777–84 [DOI] [PubMed] [Google Scholar]
- 138. Ballini A, Dipalma G, Isacco CG, Boccellino M, Di Domenico M, Santacroce L, Nguyễn KCD, Scacco S, Calvani M, Boddi A, Corcioli F, Quagliuolo L, Cantore S, Martelli FS, Inchingolo F. Oral microbiota and immune system crosstalk: a translational research. Biology 2020;9:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Charitos IA, D’Agostino D, Topi S, Bottalico L. 40 years of helicobacter pylori: a revolution in biomedical thought. Gastroenterol Insights 2021;12:111–35 [Google Scholar]
- 140. Collado MC, Meriluoto J, Salminen S. Development of new probiotics by strain combinations: is it possible to improve the adhesion to intestinal mucus? J Dairy Sci 2007;90:2710–6 [DOI] [PubMed] [Google Scholar]
- 141. Posa F, Di Benedetto A, Colaianni G, Cavalcanti-Adam EA, Brunetti G, Porro C, Trotta T, Grano M, Mori G. Vitamin D effects on osteoblastic differentiation of mesenchymal stem cells from dental tissues. Stem Cells Int 2016;2016:9150819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Santacroce L, Inchingolo F, Topi S, Del Prete R, Di Cosola M, Charitos IA, Montagnani M. Potential beneficial role of probiotics on the outcome of COVID-19 patients: an evolving perspective. Diabetes Metab Syndr 2021;15:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Slomka V, Herrero ER, Boon N, Bernaerts K, Trivedi HM, Daep C, Quirynen M, Teughels W. Oral prebiotics and the influence of environmental conditions in vitro. J Periodontol 2018;89:708–17 [DOI] [PubMed] [Google Scholar]