Abstract
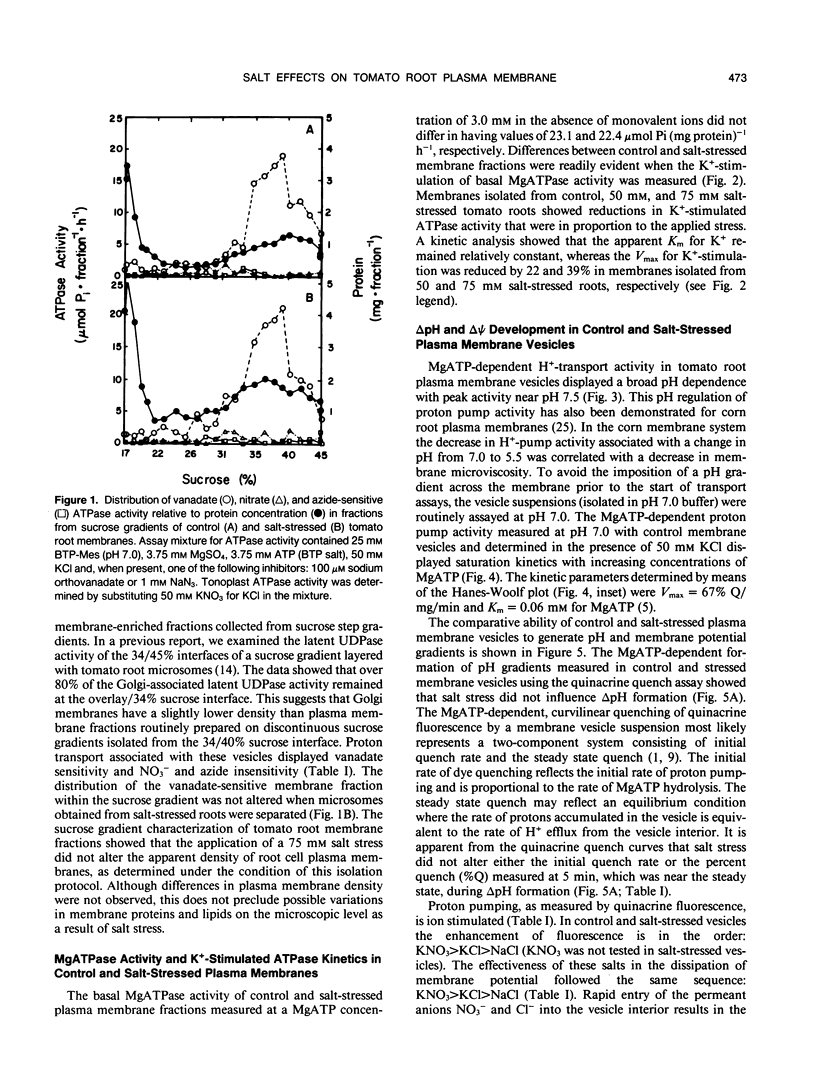
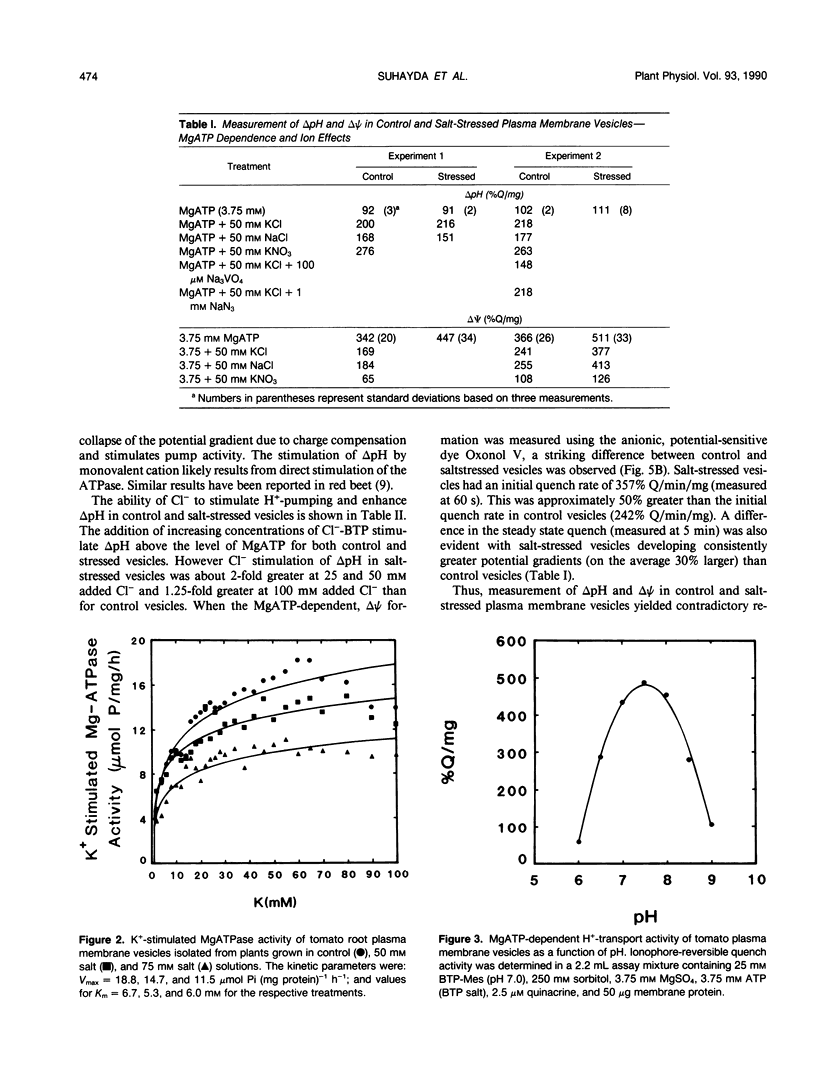
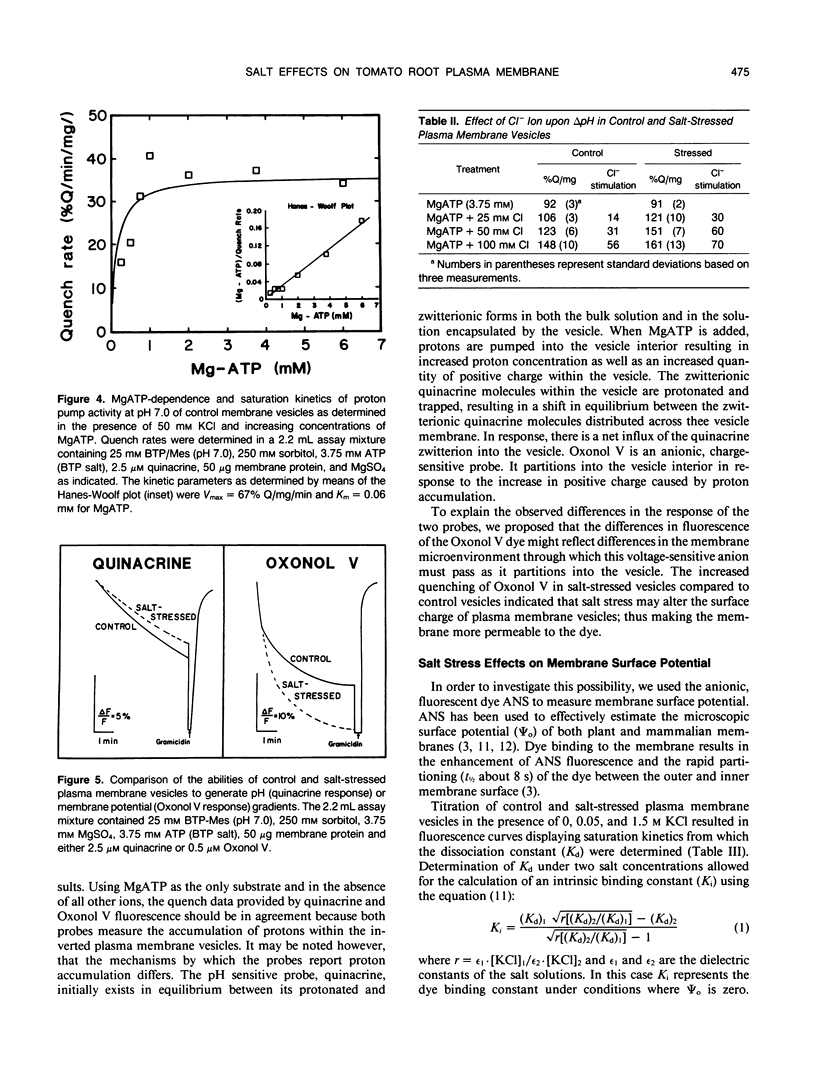
Salinity-induced alterations in tomato (Lypersicon esculentum Mill. cv Heinz 1350) root plasma membrane properties were studied and characterized using a membrane vesicle system. Equivalent rates of MgATP-dependent H+-transport activity were measured by quinacrine fluorescence (ΔpH) in plasma membrane vesicles isolated from control or salt-stressed (75 millimolar salt) tomato roots. However, when bis-[3-phenyl-5-oxoisoxazol-4-yl] pentamethine was used to measure MgATP-dependent membrane potential (ΔΨ) formation, salt-stressed vesicles displayed a 50% greater initial quench rate and a 30% greater steady state quench than control vesicles. This differential probe response suggested a difference in surface properties between control and salt-stressed membranes. Fluorescence titration of vesicles with the surface potential probe, 8-anilino-1-napthalenesulphonic acid (ANS) provided dissociation constants (Kd) of 120 and 76 micromolar for dye binding to control and salt-stressed vesicles, respectively. Membrane surface potentials (Ψo) of−26.0 and −13.7 millivolts were calculated for control and salt-stressed membrane vesicles from the measured Kd values and the calculated intrinsic affinity constant, Ki. The concentration of cations and anions at the surface of control and salt-stressed membranes was estimated using Ψo values and the Boltzmann equation. The observed difference in membrane surface electrostatic properties was consistent with the measured differences in K+-stimulated kinetics of ATPase activity between control and salt-stressed vesicles and by the differential ability of Cl− ions to stimulate H+-transport activity. Salinity-induced changes in plasma membrane electrostatic properties may influence ion transport across the plasma membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briskin D. P., Thornley W. R., Wyse R. E. Membrane transport in isolated vesicles from sugarbeet taproot : I. Isolation and characterization of energy-dependent, h-transporting vesicles. Plant Physiol. 1985 Aug;78(4):865–870. doi: 10.1104/pp.78.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu V. C., Mouring D., Watson B. D., Haynes D. H. Measurement of surface potential and surface charge densities of sarcoplasmic reticulum membranes. J Membr Biol. 1980 Sep 30;56(2):121–132. doi: 10.1007/BF01875963. [DOI] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- Deleers M. Cationic atmosphere and cation competition binding at negatively charged membranes: pathological implications of aluminum. Res Commun Chem Pathol Pharmacol. 1985 Aug;49(2):277–294. [PubMed] [Google Scholar]
- Dupont F. M. Variable Effects of Nitrate on ATP-Dependent Proton Transport by Barley Root Membranes. Plant Physiol. 1987 Jun;84(2):526–534. doi: 10.1104/pp.84.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düzgünes N., Nir S., Wilschut J., Bentz J., Newton C., Portis A., Papahadjopoulos D. Calcium- and magnesium-induced fusion of mixed phosphatidylserine/phosphatidylcholine vesicles: effect of ion binding. J Membr Biol. 1981 Apr 15;59(2):115–125. doi: 10.1007/BF01875709. [DOI] [PubMed] [Google Scholar]
- Eraso P., Cid A., Serrano R. Tight control of the amount of yeast plasma membrane ATPase during changes in growth conditions and gene dosage. FEBS Lett. 1987 Nov 16;224(1):193–197. doi: 10.1016/0014-5793(87)80446-5. [DOI] [PubMed] [Google Scholar]
- Giannini J. L., Briskin D. P. Proton Transport in Plasma Membrane and Tonoplast Vesicles from Red Beet (Beta vulgaris L.) Storage Tissue : A Comparative Study of Ion Effects on DeltapH and DeltaPsi. Plant Physiol. 1987 Jul;84(3):613–618. doi: 10.1104/pp.84.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini J. L., Gildensoph L. H., Briskin D. P. Selective production of sealed plasma membrane vesicles from red beet (Beta vulgaris L.) storage tissue. Arch Biochem Biophys. 1987 May 1;254(2):621–630. doi: 10.1016/0003-9861(87)90145-7. [DOI] [PubMed] [Google Scholar]
- Gibrat R., Romieu C., Grignon C. A procedure for estimating the surface potential of charged or neutral membranes with 8-anilino-1-naphthalenesulphonate probe. Adequacy of the Gouy-Chapman model. Biochim Biophys Acta. 1983 Dec 21;736(2):196–202. doi: 10.1016/0005-2736(83)90284-5. [DOI] [PubMed] [Google Scholar]
- Gunn R. B. Co- and counter-transport mechanisms in cell membranes. Annu Rev Physiol. 1980;42:249–259. doi: 10.1146/annurev.ph.42.030180.001341. [DOI] [PubMed] [Google Scholar]
- Honig B. H., Hubbell W. L., Flewelling R. F. Electrostatic interactions in membranes and proteins. Annu Rev Biophys Biophys Chem. 1986;15:163–193. doi: 10.1146/annurev.bb.15.060186.001115. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplified method for analysis of inorganic phosphate in the presence of interfering substances. Anal Biochem. 1978 Jan;84(1):164–172. doi: 10.1016/0003-2697(78)90495-5. [DOI] [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]


