Abstract
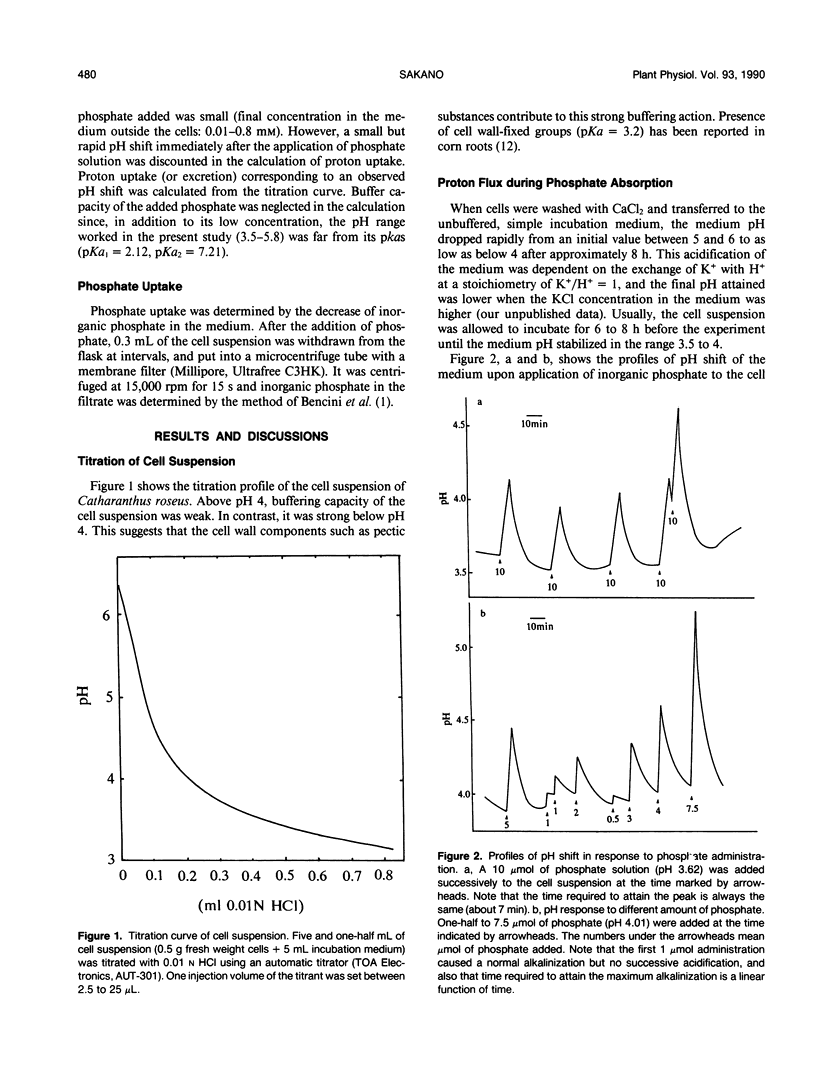
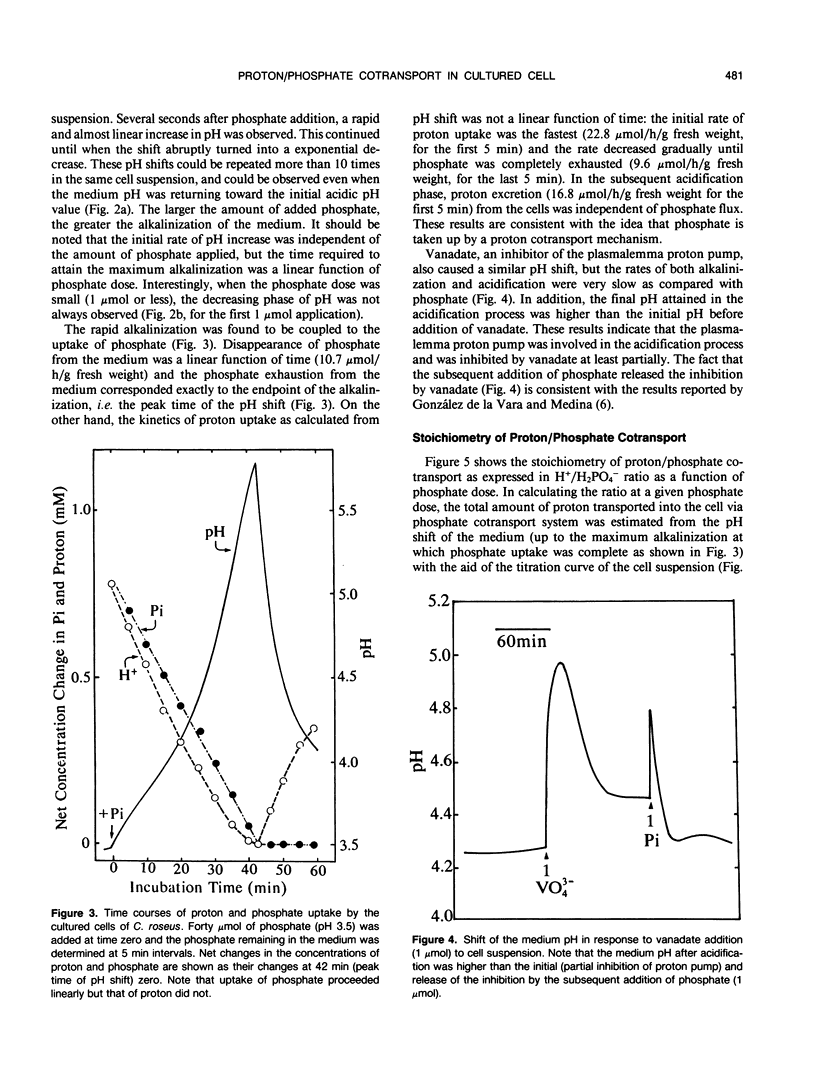
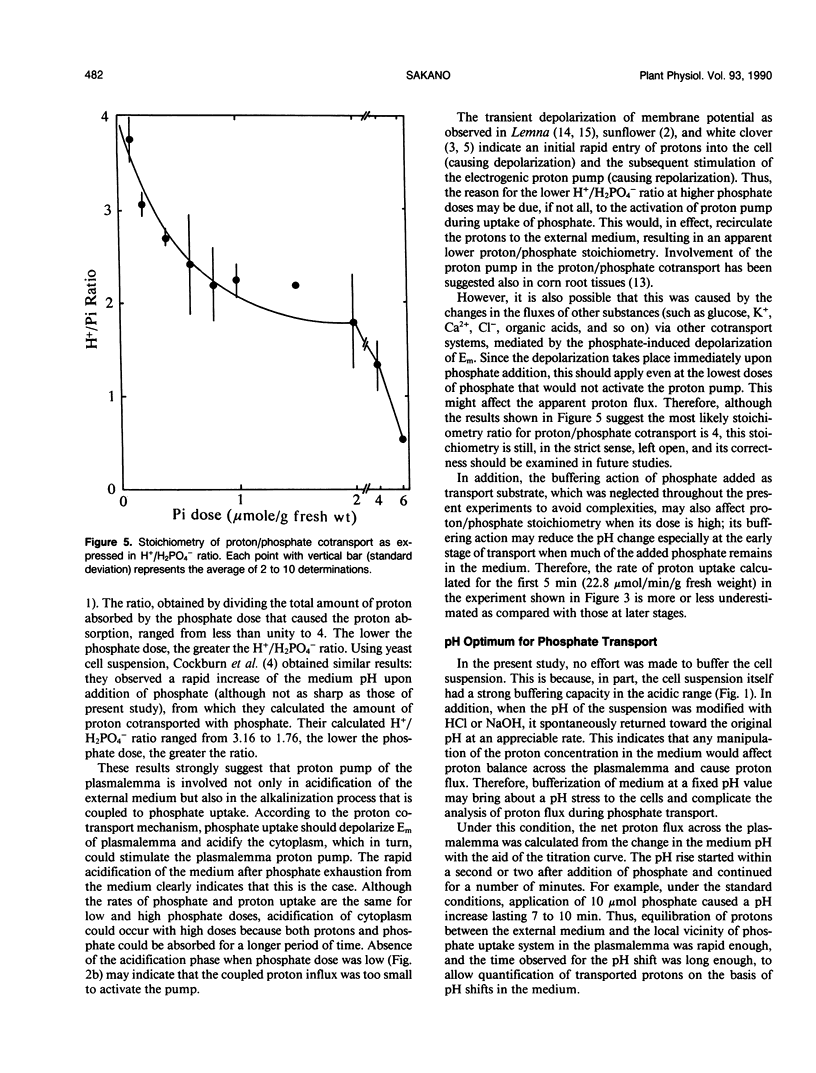
Upon absorption of phosphate, cultured cells of Catharanthus roseus (L.) G. Don caused a rapid alkalinization of the medium in which they were suspended. The alkalinization continued until the added phosphate was completely exhausted from the medium, at which time the pH of the medium started to drop sharply toward the original pH value. Phosphate exposure caused the pH of the medium to increase from pH 3.5 to values as high as 5.8, while the rate of phosphate uptake was constant throughout (10-17 micromoles per hour per gram fresh weight). This indicates that no apparent pH optimum exists for the phosphate uptake by the cultured cells. The amount of protons cotransported with phosphate was calculated from the observed pH change up to the maximum alkalinization and the titration curve of the cell suspension. Proton/phosphate transport stoichiometry ranged from less than unity to 4 according to the amount of phosphate applied. At low phosphate doses, the stoichiometries were close to 4, while at high phosphate doses, smaller stoichiometries were observed. This suggests that, at high phosphate doses, activation of the proton pump is induced by the longer lasting proton influx acidifying the cytoplasm. The increased H+ efflux due to the proton pump could partially compensate protons taken up via the proton-phosphate cotransport system. Thus, the H+/H2PO4− stoichiometry of the cotransport is most likely to be 4.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bencini D. A., Wild J. R., O'Donovan G. A. Linear one-step assay for the determination of orthophosphate. Anal Biochem. 1983 Jul 15;132(2):254–258. doi: 10.1016/0003-2697(83)90004-0. [DOI] [PubMed] [Google Scholar]
- Cockburn M., Earnshaw P., Eddy A. A. The stoicheiometry of the absorption of protons with phosphate and L-glutamate by yeasts of the genus Saccharomyces. Biochem J. 1975 Mar;146(3):705–712. doi: 10.1042/bj1460705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Ion transport in isolated protoplasts from tobacco suspension cells: I. General characteristics. Plant Physiol. 1979 Jan;63(1):183–190. doi: 10.1104/pp.63.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentenac H., Grignon C. Effect of pH on Orthophosphate Uptake by Corn Roots. Plant Physiol. 1985 Jan;77(1):136–141. doi: 10.1104/pp.77.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaud J. B., Davidian J. C., Sentenac H., Soler A., Grignon C. H Cotransports in Corn Roots as Related to the Surface pH Shift Induced by Active H Excretion. Plant Physiol. 1988 Dec;88(4):1469–1473. doi: 10.1104/pp.88.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich-Eberius C. I., Novacky A., Fischer E., Lüttge U. Relationship between Energy-dependent Phosphate Uptake and the Electrical Membrane Potential in Lemna gibba G1. Plant Physiol. 1981 Apr;67(4):797–801. doi: 10.1104/pp.67.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Vara L. E., Medina G. Effects of Inorganic Phosphate on the Plasma Membrane H-ATPase from Red Beet (Beta vulgaris L.). Plant Physiol. 1988 Dec;88(4):1073–1076. doi: 10.1104/pp.88.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]


