Abstract
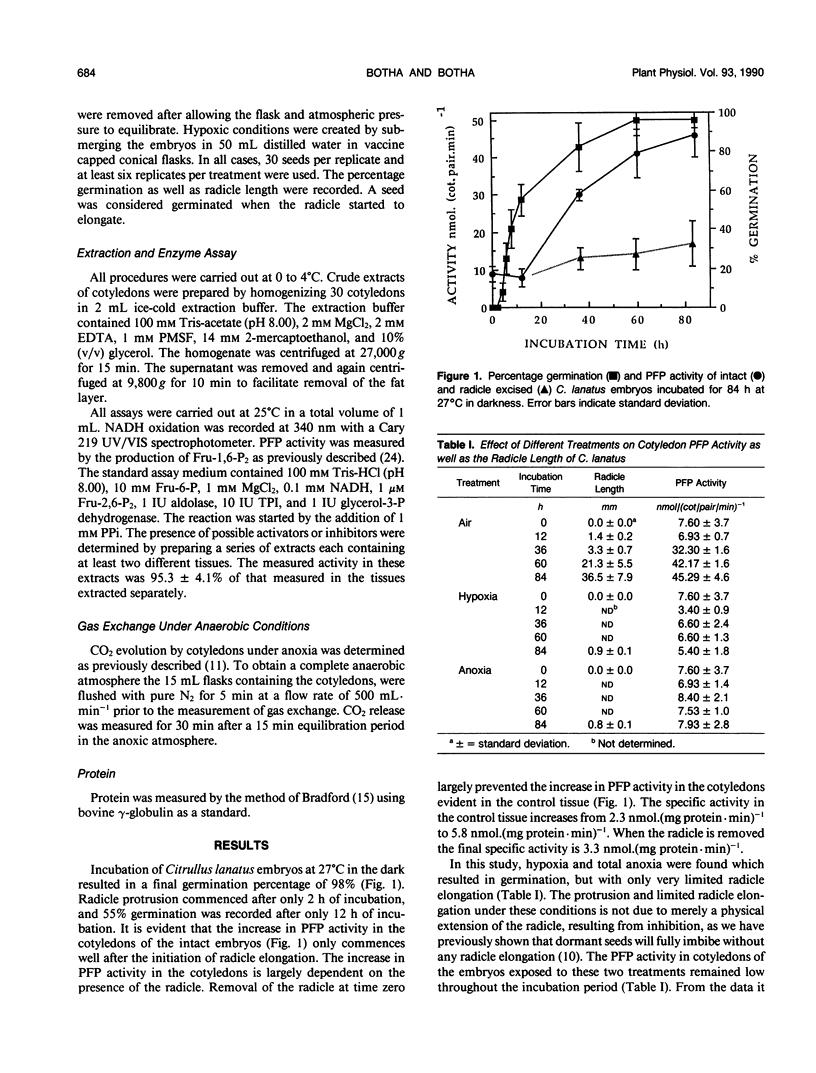
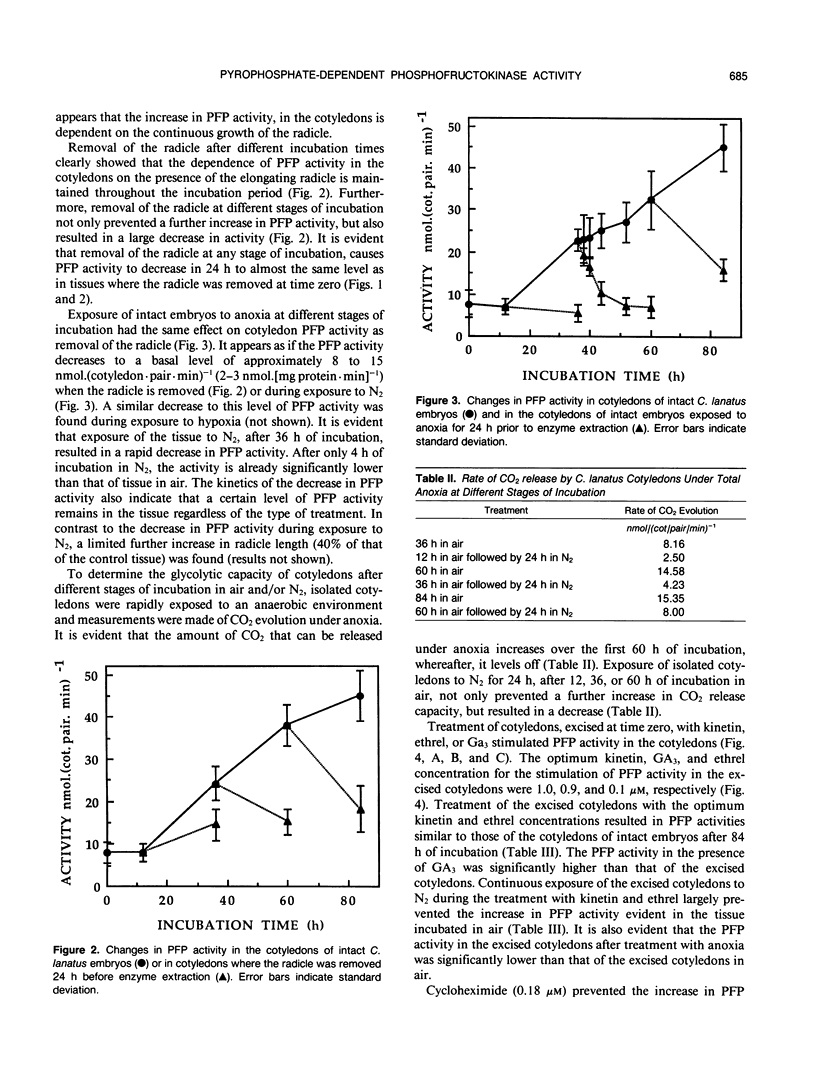
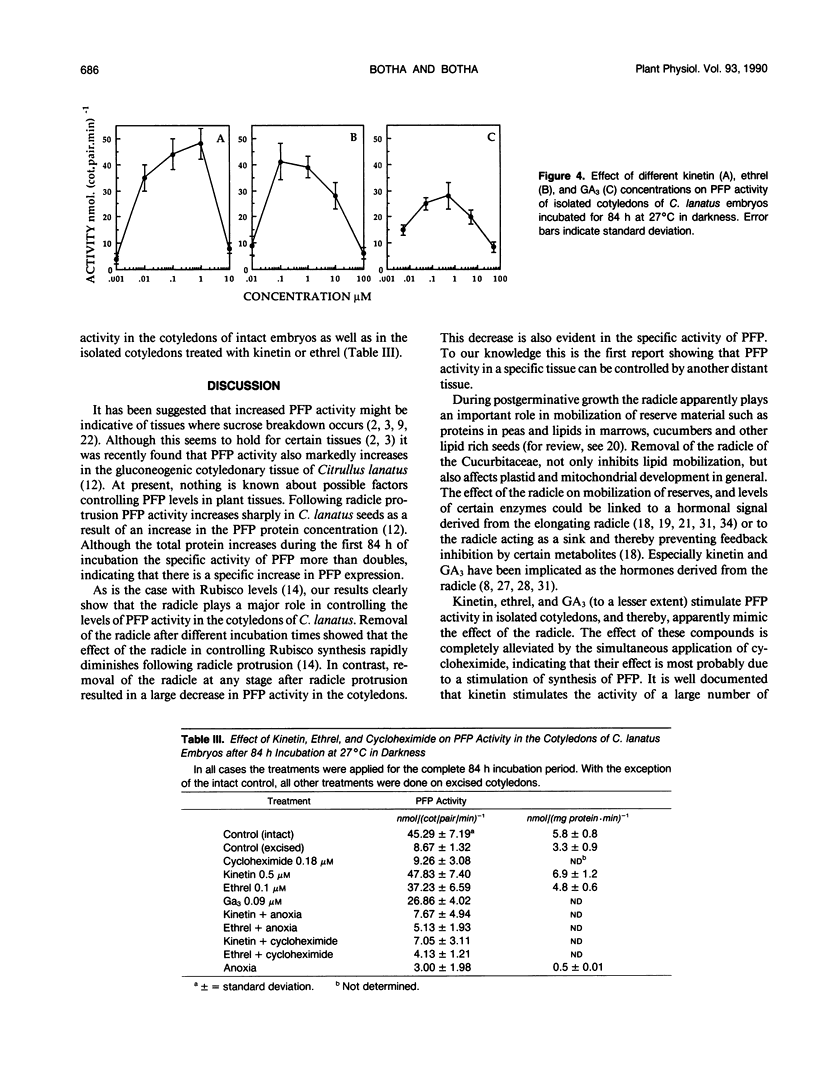
After initiation of radicle elongation, the pyrophosphate:d-fructose-6-phosphate 1-phosphotransferase (PFP) activity sharply increases in the cotyledons of Citrullus lanatus. Removal of the radicle early during incubation prevents the increase in PFP activity in the cotyledons evident in the control. Removal of the radicle at any stage after germination results in a decrease in PFP activity in the cotyledons. Application of kinetin (0.5 micromolar) or 2-chlorophosphonic acid (0.1 micromolar) to isolated cotyledons replaces the effect of the radicle. Gibberellic acid (0.09 micromolar GA3) also partially mimics the presence of the radicle. Anaerobic conditions, as well as cycloheximide application (0.18 micromolar) to intact embryos or to kinetin and ethrel treated isolated cotyledons prevent the increase in PFP activity evident in the control.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ap Rees T., Thomas S. M., Fuller W. A., Chapman B. Location of gluconeogenesis from phosphoenolpyruvate in cotyledons of Cucurbita pepo. Biochim Biophys Acta. 1975 Mar 14;385(1):145–156. doi: 10.1016/0304-4165(75)90082-3. [DOI] [PubMed] [Google Scholar]
- Barker J., Khan M. A., Solomos T. Mechanism of the Pasteur effect. Nature. 1966 Jul 30;211(5048):547–548. doi: 10.1038/211547a0. [DOI] [PubMed] [Google Scholar]
- Bertagnolli B. L., Younathan E. S., Voll R. J., Cook P. F. Kinetic studies on the activation of pyrophosphate-dependent phosphofructokinase from mung bean by fructose 2,6-bisphosphate and related compounds. Biochemistry. 1986 Aug 12;25(16):4682–4687. doi: 10.1021/bi00364a034. [DOI] [PubMed] [Google Scholar]
- Bertagnolli B. L., Younathan E. S., Voll R. J., Pittman C. E., Cook P. F. Carbohydrate substrate specificity of bacterial and plant pyrophosphate-dependent phosphofructokinases. Biochemistry. 1986 Aug 12;25(16):4674–4681. doi: 10.1021/bi00364a033. [DOI] [PubMed] [Google Scholar]
- Botha F. C., Small J. G. Comparison of the Activities and Some Properties of Pyrophosphate and ATP Dependent Fructose-6-Phosphate 1-Phosphotransferases of Phaseolus vulgaris Seeds. Plant Physiol. 1987 Apr;83(4):772–777. doi: 10.1104/pp.83.4.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carnal N. W., Black C. C. Pyrophosphate-dependent 6-phosphofructokinase, a new glycolytic enzyme in pineapple leaves. Biochem Biophys Res Commun. 1979 Jan 15;86(1):20–26. doi: 10.1016/0006-291x(79)90376-0. [DOI] [PubMed] [Google Scholar]
- Chalmers D. J., Rowan K. S. The climacteric in ripening tomato fruit. Plant Physiol. 1971 Sep;48(3):235–240. doi: 10.1104/pp.48.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Akazawa T. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol. 1986 Aug;81(4):1008–1013. doi: 10.1104/pp.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Kruger N. J., Beevers H. Kinetic properties of pyrophosphate:fructose-6-phosphate phosphotransferase from germinating castor bean endosperm. Plant Physiol. 1984 Feb;74(2):395–401. doi: 10.1104/pp.74.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maesen F. P., Davies B. I., Teengs J. P., Baur C. Quinolone antimicrobial agents in acute exacerbations of chronic bronchitis. Eur J Respir Dis Suppl. 1986;146:585–590. [PubMed] [Google Scholar]
- Matters G. L., Scandalios J. G. Changes in plant gene expression during stress. Dev Genet. 1986;7(4):167–175. doi: 10.1002/dvg.1020070402. [DOI] [PubMed] [Google Scholar]
- Sabularse D. C., Anderson R. L. D-Fructose 2,6-bisphosphate: a naturally occurring activator for inorganic pyrophosphate:D-fructose-6-phosphate 1-phosphotransferase in plants. Biochem Biophys Res Commun. 1981 Dec 15;103(3):848–855. doi: 10.1016/0006-291x(81)90888-3. [DOI] [PubMed] [Google Scholar]
- Sabularse D. C., Anderson R. L. Inorganic pyrophosphate: D-fructose-6-phosphate 1-phosphotransferase in mung beans and its activation by D-fructose 1,6-bisphosphate and D-glucose 1, 6-bisphosphate. Biochem Biophys Res Commun. 1981 Jun;100(4):1423–1429. doi: 10.1016/0006-291x(81)90677-x. [DOI] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Similarities between the Actions of Ethylene and Cyanide in Initiating the Climacteric and Ripening of Avocados. Plant Physiol. 1974 Oct;54(4):506–511. doi: 10.1104/pp.54.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ap Rees T., Green J. H., Wilson P. M. Pyrophosphate:fructose 6-phosphate 1-phosphotransferase and glycolysis in non-photosynthetic tissues of higher plants. Biochem J. 1985 Apr 1;227(1):299–304. doi: 10.1042/bj2270299. [DOI] [PMC free article] [PubMed] [Google Scholar]


