Abstract
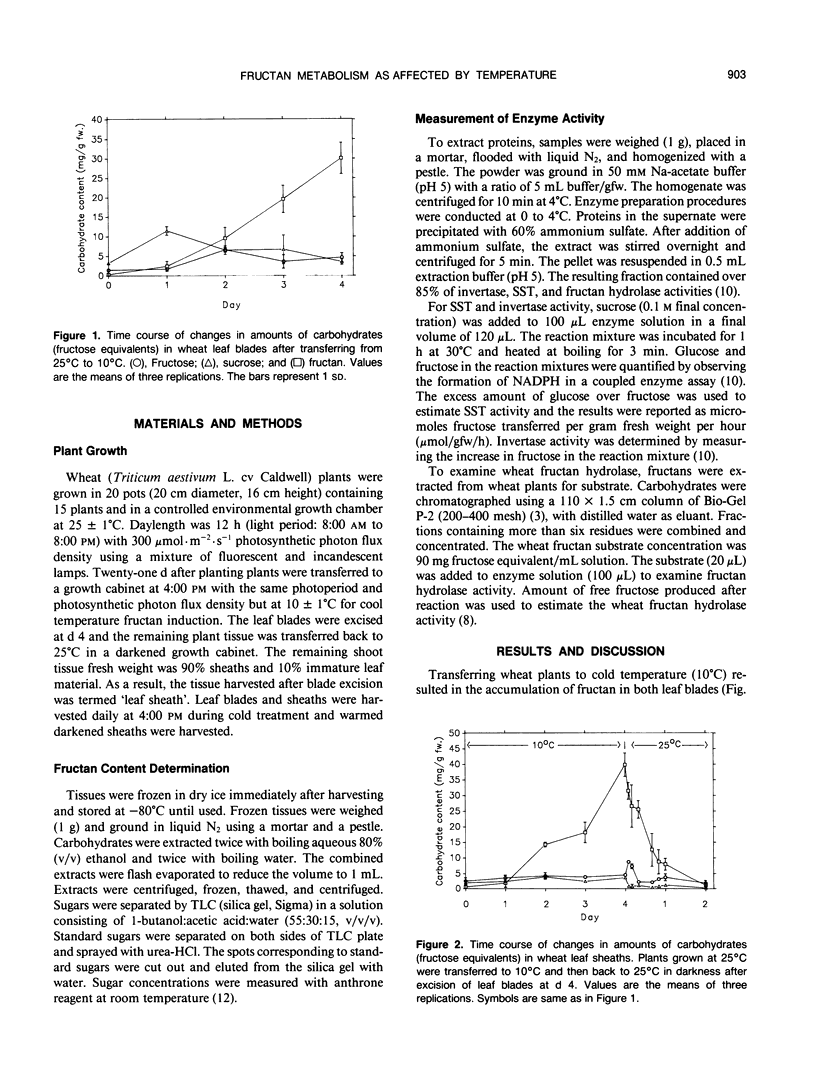
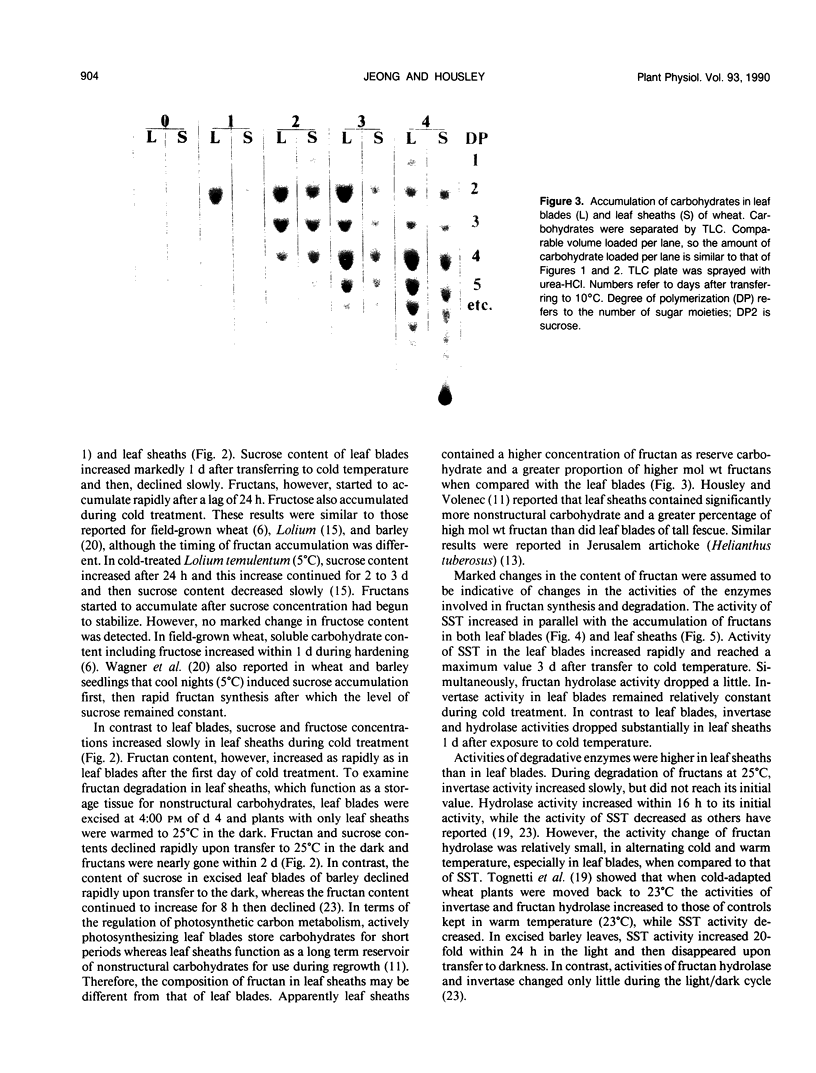
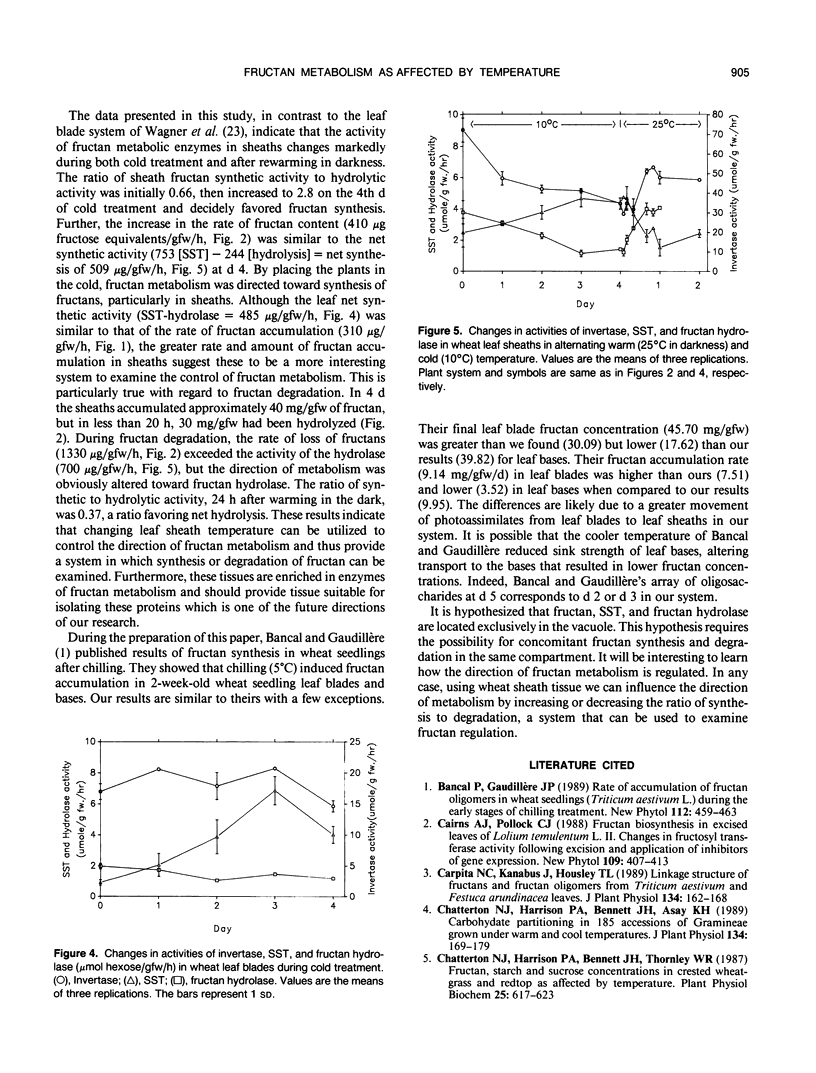
The objective of this research was to develop a system in which the direction of fructan metabolism could be controlled. Three-week-old wheat seedlings (Triticum aestivum L. cv Caldwell) grown at 25°C were transferred to cold temperature (10°C) to induce fructan synthesis and then were transferred to continuous darkness at 25°C after defoliation and fructan degradation monitored. The total fructan content increased significantly 1 day after transferring from 25°C to 10°C in both leaf blades and the remainder of the shoot tissue, 90% of which was leaf sheath tissue. Leaf sheaths contained higher concentrations of fructan and greater portions of high molecular weight fructan than did leaf blades. Fructan content in leaf sheaths declined rapidly and was gone completely within 48 hours following transfer to 25°C in darkness. In leaf blades the invertase activity fluctuated during cold treatment. The activity of sucrose:sucrose fructosyl transferase increased markedly during cold treatment, while fructan hydrolase activity decreased slightly. In leaf sheaths, however, the activity of invertase decreased rapidly upon transfer to cold temperature and remained low. Trends in sucrose:sucrose fructosyl transferase and hydrolase activity in sheaths were the same as those of leaf blades. Sheath invertase and hydrolase activity increased when plants were transferred back to darkness at 25°C, while sucrose:sucrose fructosyl transferase activity decreased. These results indicate that changing leaf sheath temperature can be utilized to control the direction of fructan metabolism and thus provide a system in which the synthesis or degradation of fructan can be examined.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Housley T. L., Daughtry C. S. Fructan Content and Fructosyltransferase Activity during Wheat Seed Growth. Plant Physiol. 1987 Jan;83(1):4–7. doi: 10.1104/pp.83.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley T. L., Volenec J. J. Fructan Content and Synthesis in Leaf Tissues of Festuca arundinacea. Plant Physiol. 1988 Apr;86(4):1247–1251. doi: 10.1104/pp.86.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., Wiemken A. Enzymology of Fructan Synthesis in Grasses: Properties of Sucrose-Sucrose-Fructosyltransferase in Barley Leaves (Hordeum vulgare L. cv Gerbel). Plant Physiol. 1987 Nov;85(3):706–710. doi: 10.1104/pp.85.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., Wiemken A., Matile P. Regulation of Fructan Metabolism in Leaves of Barley (Hordeum vulgare L. cv Gerbel). Plant Physiol. 1986 Jun;81(2):444–447. doi: 10.1104/pp.81.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Mino Y. Partial purification and properties of phleinase induced in stem base of orchardgrass after defoliation. Plant Physiol. 1985 Jul;78(3):591–595. doi: 10.1104/pp.78.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]



