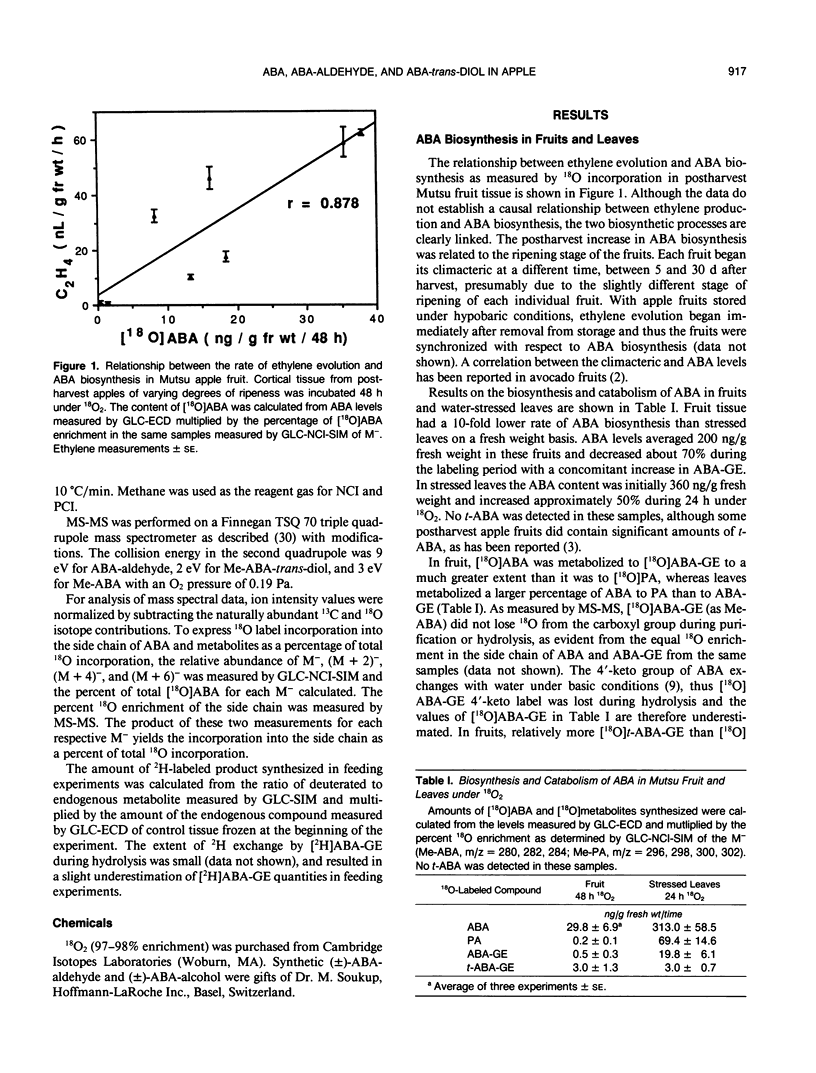
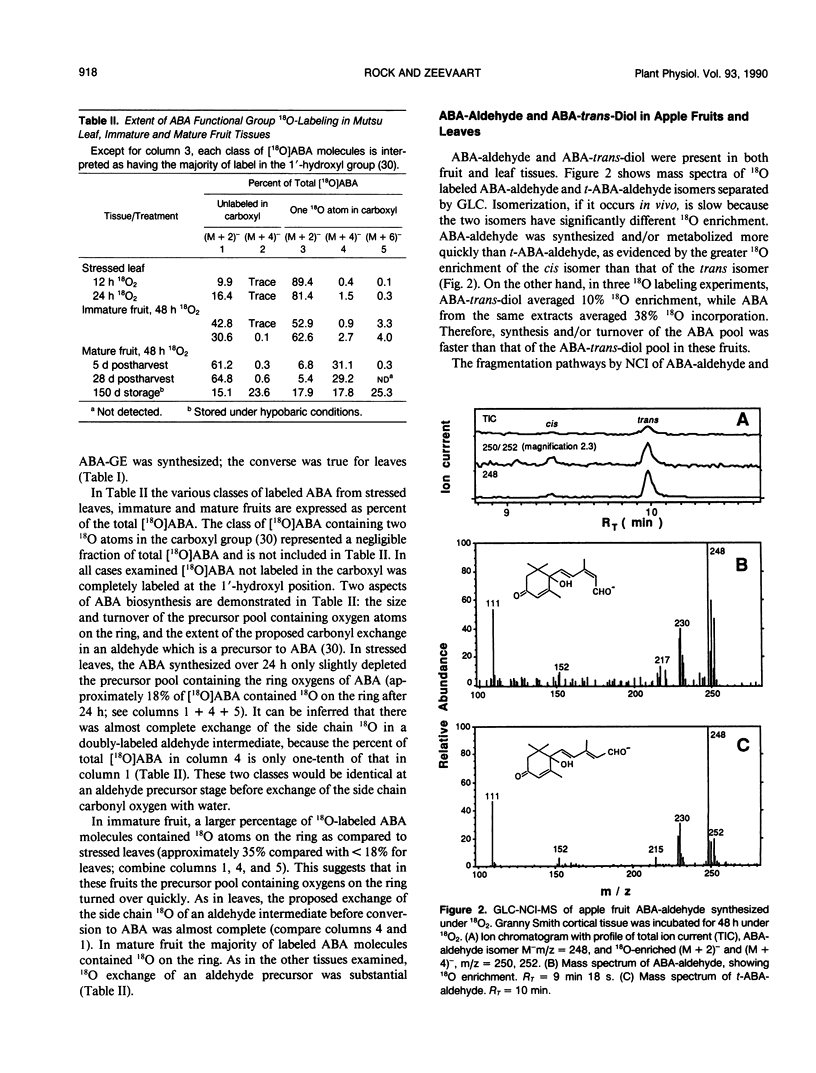
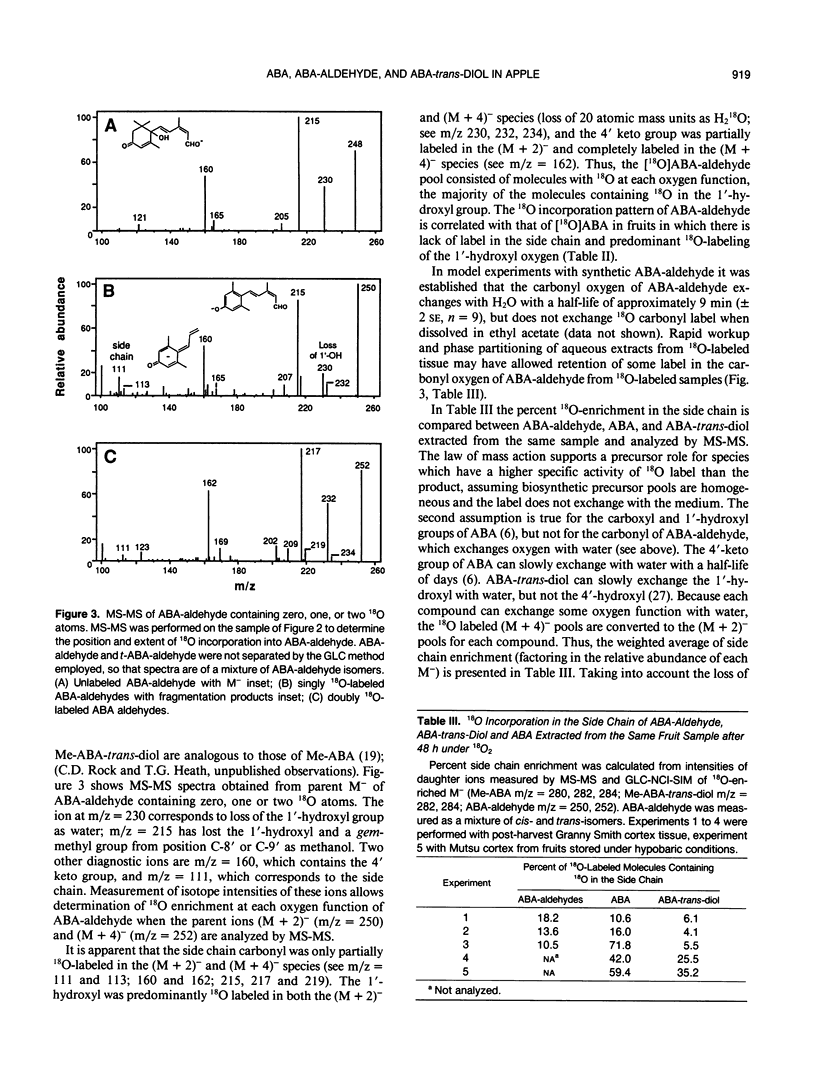
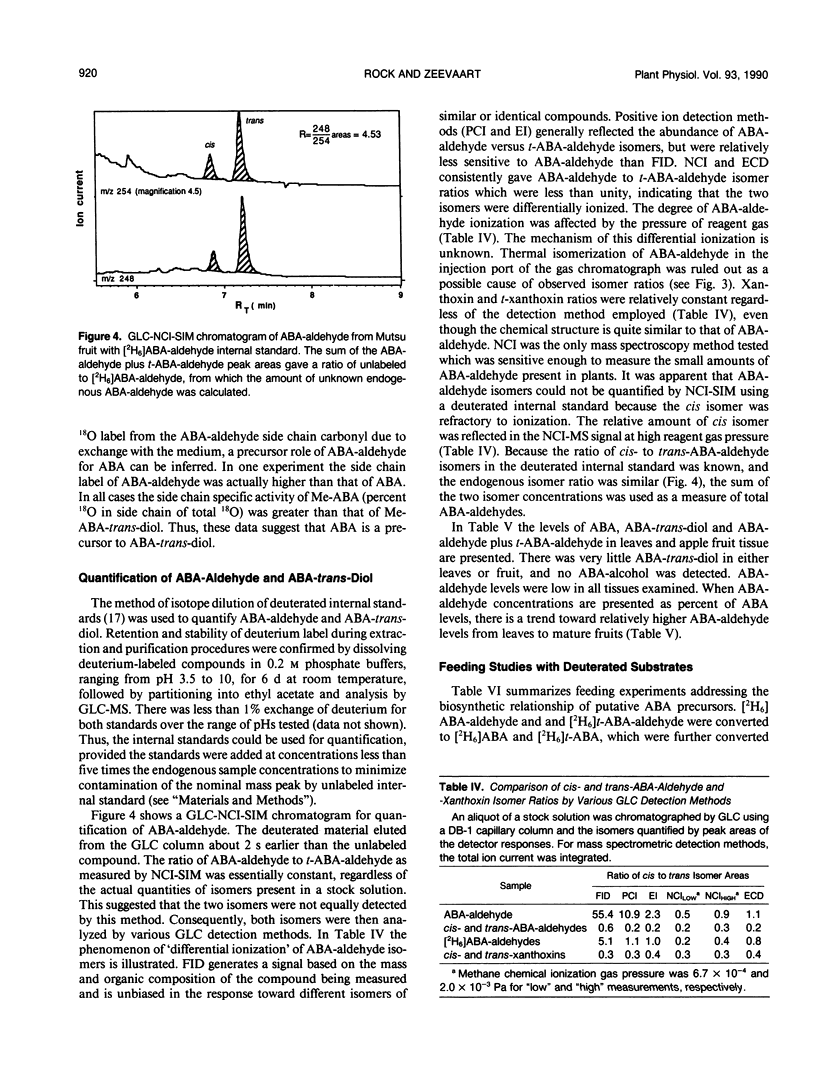
Abstract
Previous 18O labeling studies of abscisic acid (ABA) have shown that apple (Malus domestica Borkh. cv Granny Smith) fruits synthesize a majority of [18O]ABA with the label incorporated in the 1′-hydroxyl position and unlabeled in the carboxyl group (JAD Zeevaart, TG Heath, DA Gage [1989] Plant Physiol 91: 1594-1601). It was proposed that exchange of 18O in the side chain with the medium occurred at an aldehyde intermediate stage of ABA biosynthesis. We have isolated ABA-aldehyde and 1′-4′-trans-ABA-diol (ABA-trans-diol) from 18O-labeled apple fruit tissue and measured the extent and position of 18O incorporation by tandem mass spectrometry. 18O-Labeling patterns of ABA-aldehyde, ABA-trans-diol, and ABA indicate that ABA-aldehyde is a precursor to, and ABA-trans-diol a catabolite of, ABA. Exchange of 18O in the carbonyl of ABA-aldehyde can be the cause of loss of 18O from the side chain of [18O]ABA. Results of feeding experiments with deuterated substrates provide further support for the precursor-product relationship of ABA-aldehyde → ABA → ABA-trans-diol. The ABA-aldehyde and ABA-trans-diol contents of fruits and leaves were low, approximately 1 and 0.02 nanograms per gram fresh weight for ABA-aldehyde and ABA-trans-diol, respectively, while ABA levels in fruits ranged from 10 to 200 nanograms per gram fresh weight. ABA biosynthesis was about 10-fold lower in fruits than in leaves. In fruits, the majority of ABA was conjugated to β-d-glucopyranosyl abscisate, whereas in leaves ABA was mainly hydroxylated to phaseic acid. Parallel pathways for ABA and trans-ABA biosynthesis and conjugation in fruits and leaves are proposed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer G. L., Zeevaart J. A. Isolation and Quantitation of beta-d-Glucopyranosyl Abscisate from Leaves of Xanthium and Spinach. Plant Physiol. 1982 Jul;70(1):227–231. doi: 10.1104/pp.70.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K., Zeevaart J. A. Abscisic Acid Metabolism in Relation to Water Stress and Leaf Age in Xanthium strumarium. Plant Physiol. 1984 Dec;76(4):1029–1035. doi: 10.1104/pp.76.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Gage D. A., Stults J. T., Zeevaart J. A. Abscisic Acid Biosynthesis in Leaves and Roots of Xanthium strumarium. Plant Physiol. 1987 Nov;85(3):726–732. doi: 10.1104/pp.85.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Zeevaart J. A. Incorporation of oxygen into abscisic Acid and phaseic Acid from molecular oxygen. Plant Physiol. 1984 May;75(1):166–169. doi: 10.1104/pp.75.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble P. E., Mullet J. E. Inhibition of carotenoid accumulation and abscisic acid biosynthesis in fluridone-treated dark-grown barley. Eur J Biochem. 1986 Oct 1;160(1):117–121. doi: 10.1111/j.1432-1033.1986.tb09947.x. [DOI] [PubMed] [Google Scholar]
- Kende H., Hanson A. D. Relationship between Ethylene Evolution and Senescence in Morning-Glory Flower Tissue. Plant Physiol. 1976 Apr;57(4):523–527. doi: 10.1104/pp.57.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Walton D. C. Violaxanthin is an abscisic Acid precursor in water-stressed dark-grown bean leaves. Plant Physiol. 1990 Mar;92(3):551–559. doi: 10.1104/pp.92.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Walton D. C. Xanthophylls and abscisic Acid biosynthesis in water-stressed bean leaves. Plant Physiol. 1987 Dec;85(4):910–915. doi: 10.1104/pp.85.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu R. K., Walton D. C. Conversion of xanthoxin to abscisic Acid by cell-free preparations from bean leaves. Plant Physiol. 1987 Dec;85(4):916–921. doi: 10.1104/pp.85.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu R. K., Walton D. C. Xanthoxin Metabolism in Cell-free Preparations from Wild Type and Wilty Mutants of Tomato. Plant Physiol. 1988 Sep;88(1):178–182. doi: 10.1104/pp.88.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. F., Burden R. S. Xanthoxin, a recently discovered plant growth inhibitor. Proc R Soc Lond B Biol Sci. 1972 Mar 14;180(1060):317–346. doi: 10.1098/rspb.1972.0020. [DOI] [PubMed] [Google Scholar]
- Zeevaart J. A., Heath T. G., Gage D. A. Evidence for a universal pathway of abscisic Acid biosynthesis in higher plants from o incorporation patterns. Plant Physiol. 1989 Dec;91(4):1594–1601. doi: 10.1104/pp.91.4.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]


