Abstract
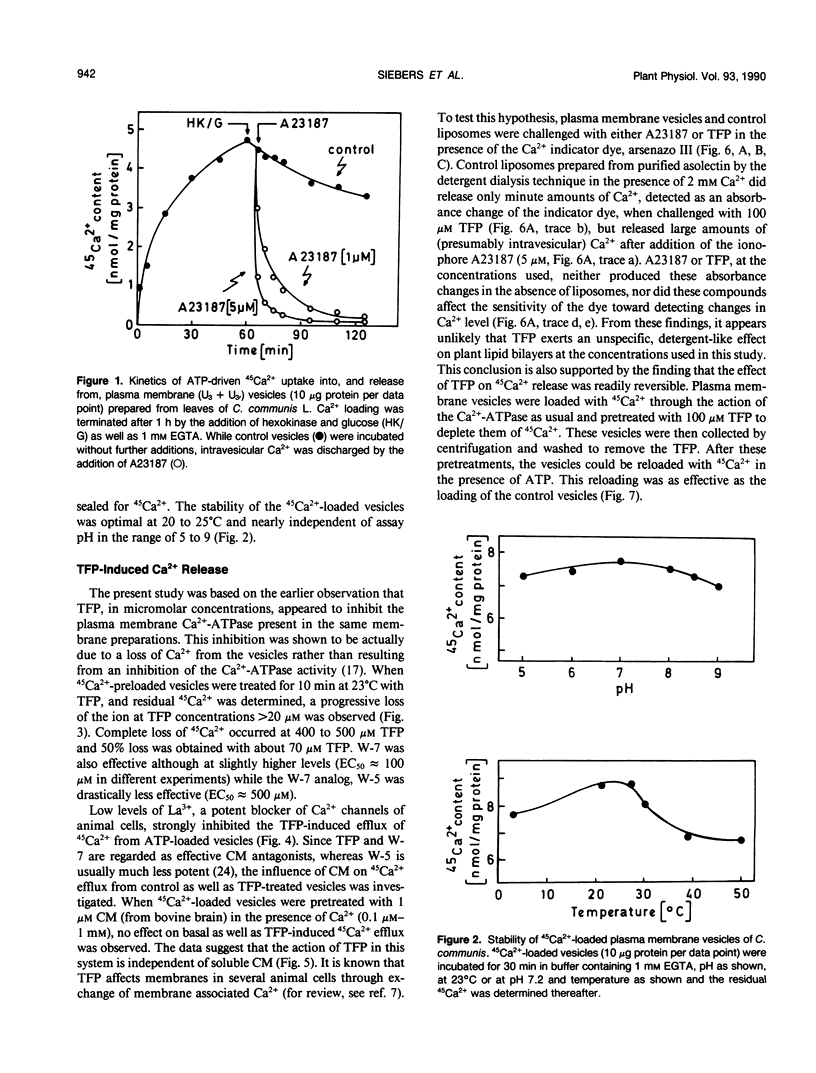
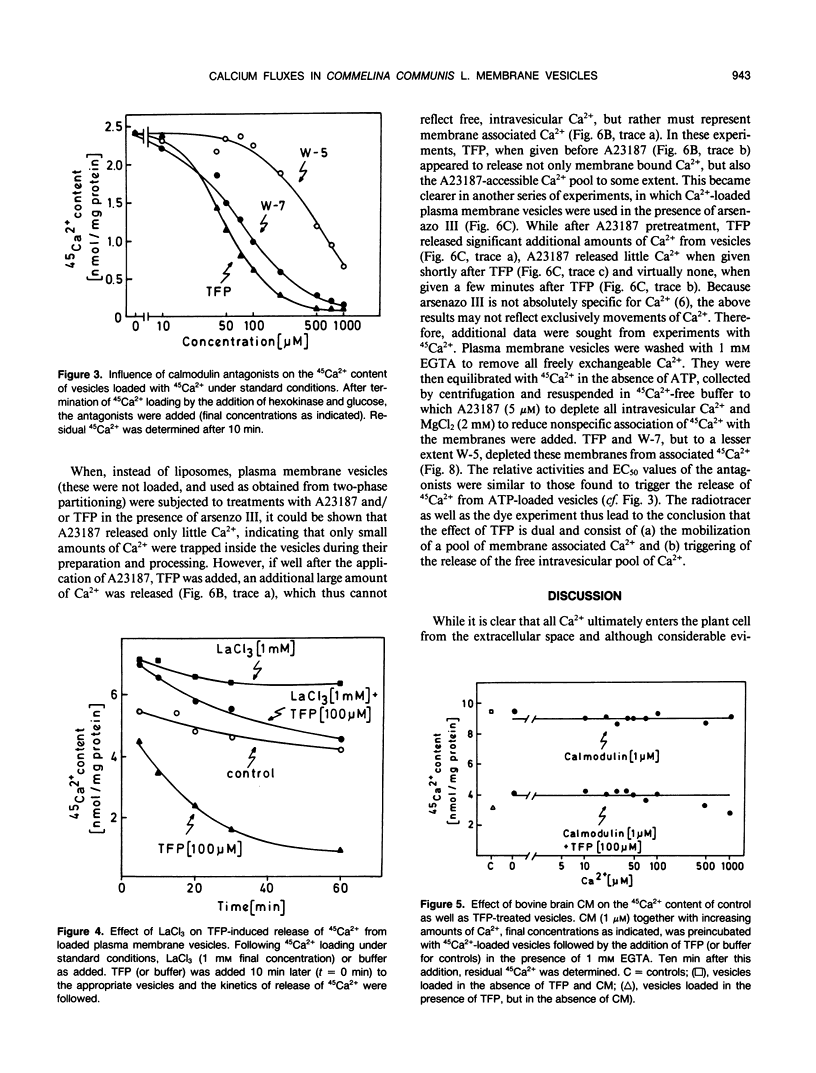
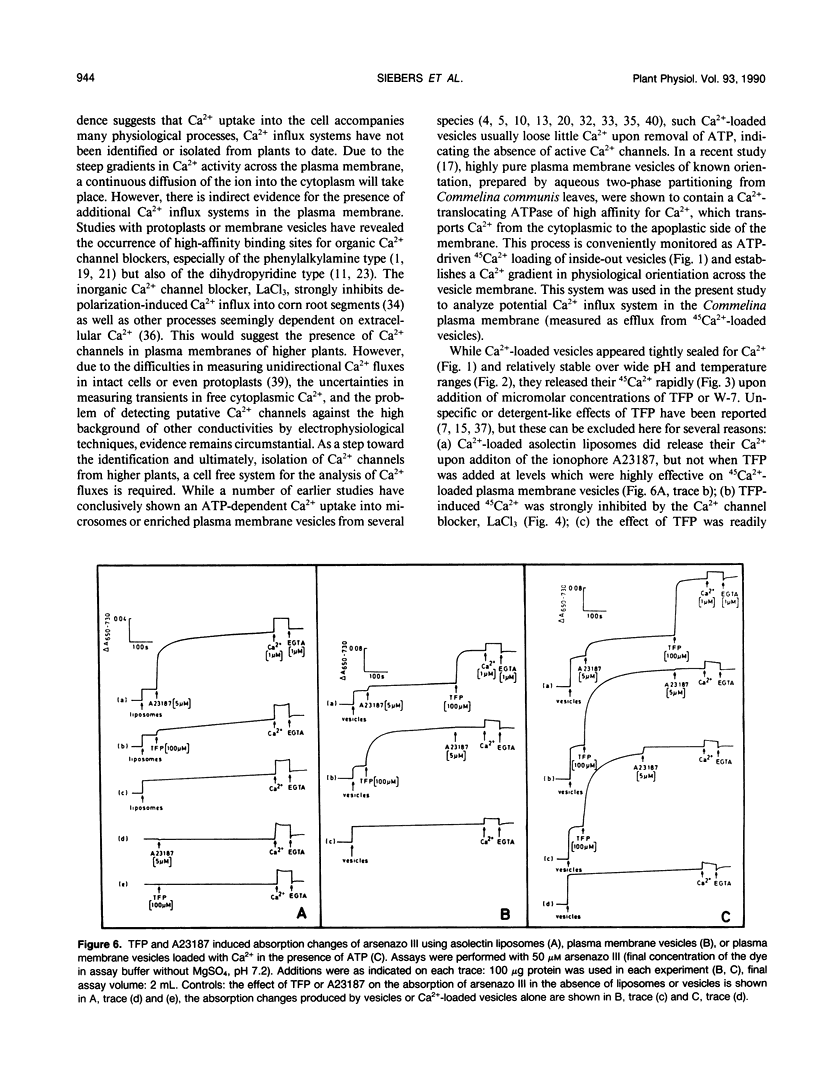
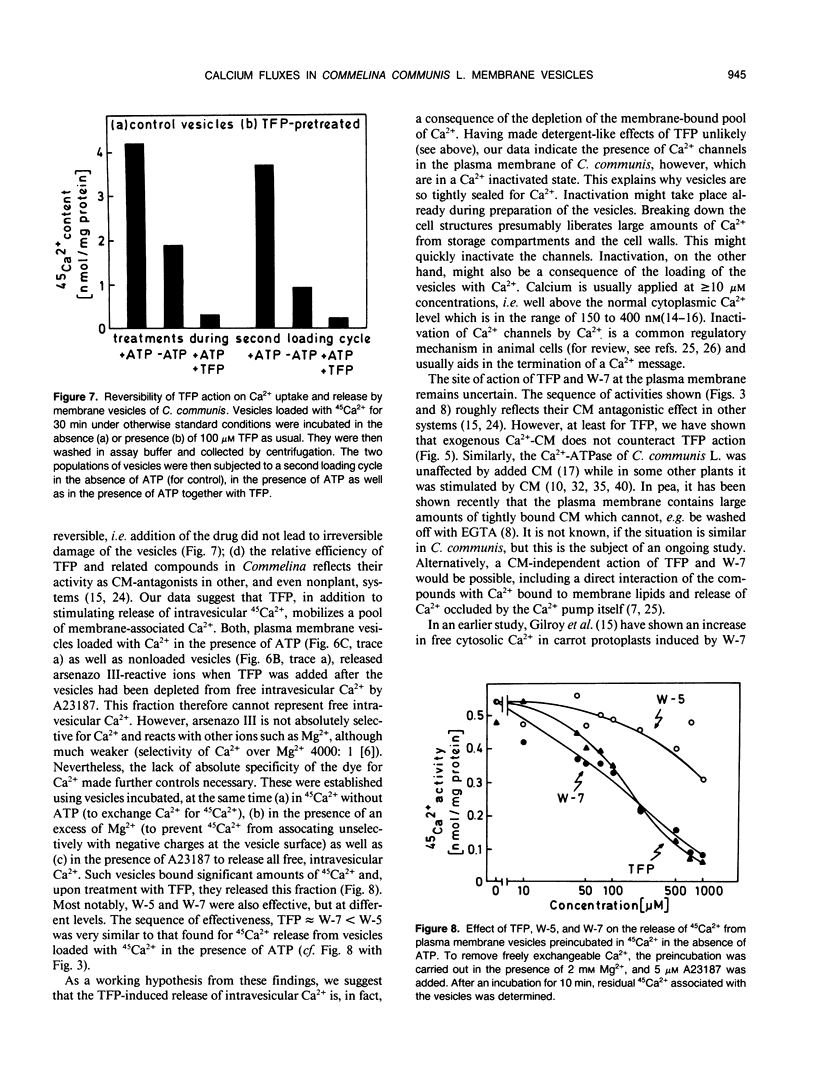
The inside-out fraction of plasma membrane-rich vesicles prepared from leaves of Commelina communis L. by aqueous twophase partitioning was loaded with 45Ca2+ through the action of the plasma membrane Ca2+-ATPase. While the Ca2+-loaded vesicles were tightly sealed, trifluoperazine (TFP) (effective concentration giving 50% of maximum effect [EC50] = 70 micromolar) and W-7 (EC50 = 100 micromolar), but to a much lesser extent, W-5 (EC50 = 500 micromolar) led to a rapid efflux of 45Ca2+ from the vesicles. This efflux could be blocked efficiently with low (<1 millimolar) concentrations of La3+, but it remained unaffected by the addition of calmodulin (CM). Further experiments with vesicles incubated in 45Ca2+ in the absence of ATP, as well as experiments performed with control liposomes and nonloaded as well as Ca2+-loaded plasma membrane vesicles using the indicator dye arsenazo III showed, that TFP and W-7 and, again to a lesser extent, W-5 mobilized a pool of membrane-bound Ca2+ from the vesicles. No indications for a detergent effect of TFP and W-7 were obtained. The EC50-values of these compounds for mobilizing membrane-associated Ca2+ (TFP = 100 micromolar, W-7 = 100 micromolar, W-5 = 500 micromolar) or for the triggering of Ca2+ release from Ca2+-loaded vesicles (see above) were very similar, suggesting a common basis of antagonist action on both processes. Our results suggest the presence of a Ca2+ channel in the plasma membrane of C. communis. The channel is obtained in a Ca2+-inactivated state after preparation and Ca2+-loading of the vesicles. The inactivation is removed by TFP or W-7, presumably due to the Ca2+-mobilizing effect of these compounds. The activated Ca2+ channel is La3+ sensitive and, in the cell, would allow for passage of Ca2+ into the cell. The possibility that TFP or W-7 act independent of CM, or through CM tightly associated with the plasma membrane, is discussed. The system described allows a cell free analysis of Ca2+ influx, displaying channel properties, in a higher plant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrejauskas E., Hertel R., Marmé D. Specific binding of the calcium antagonist [3H]verapamil to membrane fractions from plants. J Biol Chem. 1985 May 10;260(9):5411–5414. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Collinge M., Trewavas A. J. The location of calmodulin in the pea plasma membrane. J Biol Chem. 1989 May 25;264(15):8865–8872. [PubMed] [Google Scholar]
- Cook N. J., Zeilinger C., Koch K. W., Kaupp U. B. Solubilization and functional reconstitution of the cGMP-dependent cation channel from bovine rod outer segments. J Biol Chem. 1986 Dec 25;261(36):17033–17039. [PubMed] [Google Scholar]
- Dieter P., Marmé D. Calmodulin activation of plant microsomal Ca uptake. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7311–7314. doi: 10.1073/pnas.77.12.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini J. L., Ruiz-Cristin J., Briskin D. P. Calcium Transport in Sealed Vesicles from Red Beet (Beta vulgaris L.) Storage Tissue : II. Characterization of Ca Uptake into Plasma Membrane Vesicles. Plant Physiol. 1987 Dec;85(4):1137–1142. doi: 10.1104/pp.85.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S., Hughes W. A., Trewavas A. J. A Comparison between Quin-2 and Aequorin as Indicators of Cytoplasmic Calcium Levels in Higher Plant Cell Protoplasts. Plant Physiol. 1989 Jun;90(2):482–491. doi: 10.1104/pp.90.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Marmé D. ATP-dependent Ca uptake into plant membrane vesicles. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1232–1236. doi: 10.1073/pnas.75.3.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey H. J., Venis M. A., Trewavas A. J. Partial purification of a protein from maize (Zea mays) coleoptile membranes binding the Ca2+-channel antagonist verapamil. Biochem J. 1989 Jan 1;257(1):95–100. doi: 10.1042/bj2570095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Tanaka T. Naphthalenesulfonamides as calmodulin antagonists. Methods Enzymol. 1983;102:185–194. doi: 10.1016/s0076-6879(83)02019-4. [DOI] [PubMed] [Google Scholar]
- Hosey M. M., Lazdunski M. Calcium channels: molecular pharmacology, structure and regulation. J Membr Biol. 1988 Sep;104(2):81–105. doi: 10.1007/BF01870922. [DOI] [PubMed] [Google Scholar]
- Jena P. K., Reddy A. S., Poovaiah B. W. Molecular cloning and sequencing of a cDNA for plant calmodulin: signal-induced changes in the expression of calmodulin. Proc Natl Acad Sci U S A. 1989 May;86(10):3644–3648. doi: 10.1073/pnas.86.10.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K. H., Chou M., Thomas M. A., Sjolund R. D., Campbell K. P. Plant cells contain calsequestrin. J Biol Chem. 1989 Mar 15;264(8):4269–4272. [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C., De Michelis M. I. The Ca-Transport ATPase of Plant Plasma Membrane Catalyzes a nH/Ca Exchange. Plant Physiol. 1987 Apr;83(4):994–1000. doi: 10.1104/pp.83.4.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin G., Fleurat-Lessard P., Bonmort J. Effects of Compounds Affecting Calcium Channels on Phytochrome- and Blue Pigment-Mediated Pulvinar Movements of Cassia fasciculata. Plant Physiol. 1989 Jun;90(2):697–701. doi: 10.1104/pp.90.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roufogalis B. D. Phenothiazine antagonism of calmodulin: a structurally-nonspecific interaction. Biochem Biophys Res Commun. 1981 Feb 12;98(3):607–613. doi: 10.1016/0006-291x(81)91157-8. [DOI] [PubMed] [Google Scholar]
- Wrona A. F., Spanswick R. M., Aist J. R. Calcium Transport in Protoplasts Isolated from ml-o Barley Isolines Resistant and Susceptible to Powdery Mildew. Plant Physiol. 1988 Dec;88(4):1157–1162. doi: 10.1104/pp.88.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]


