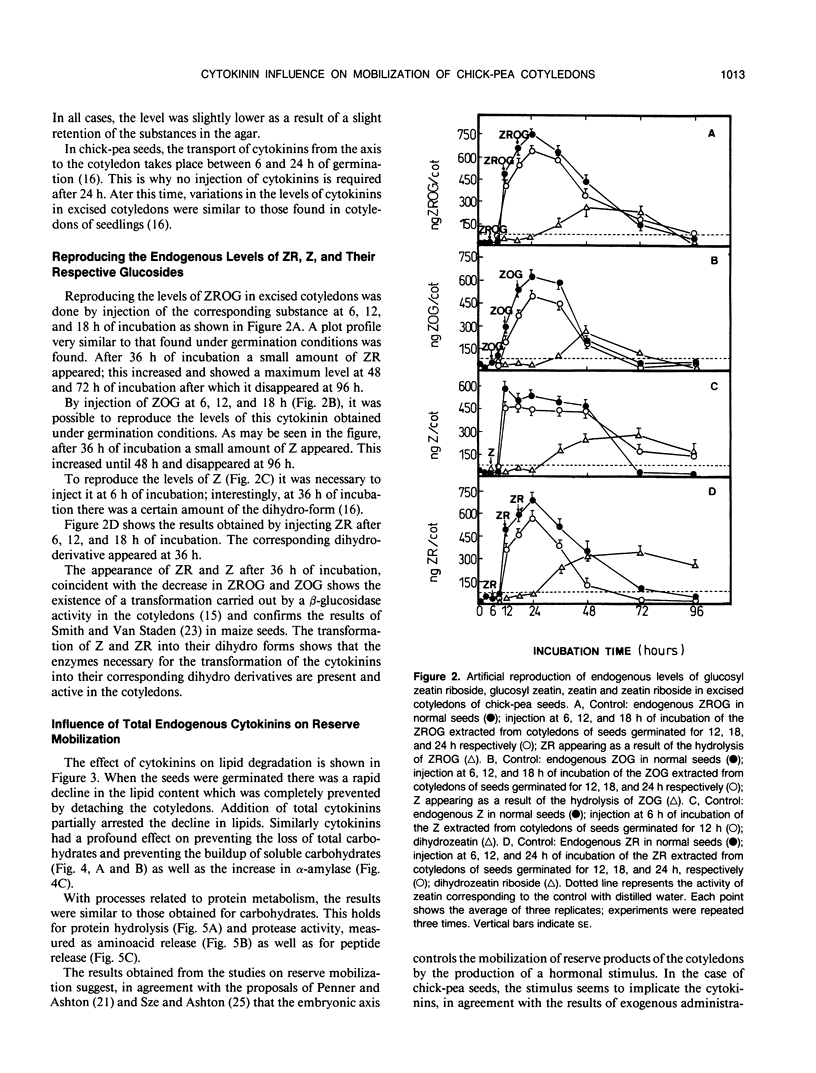
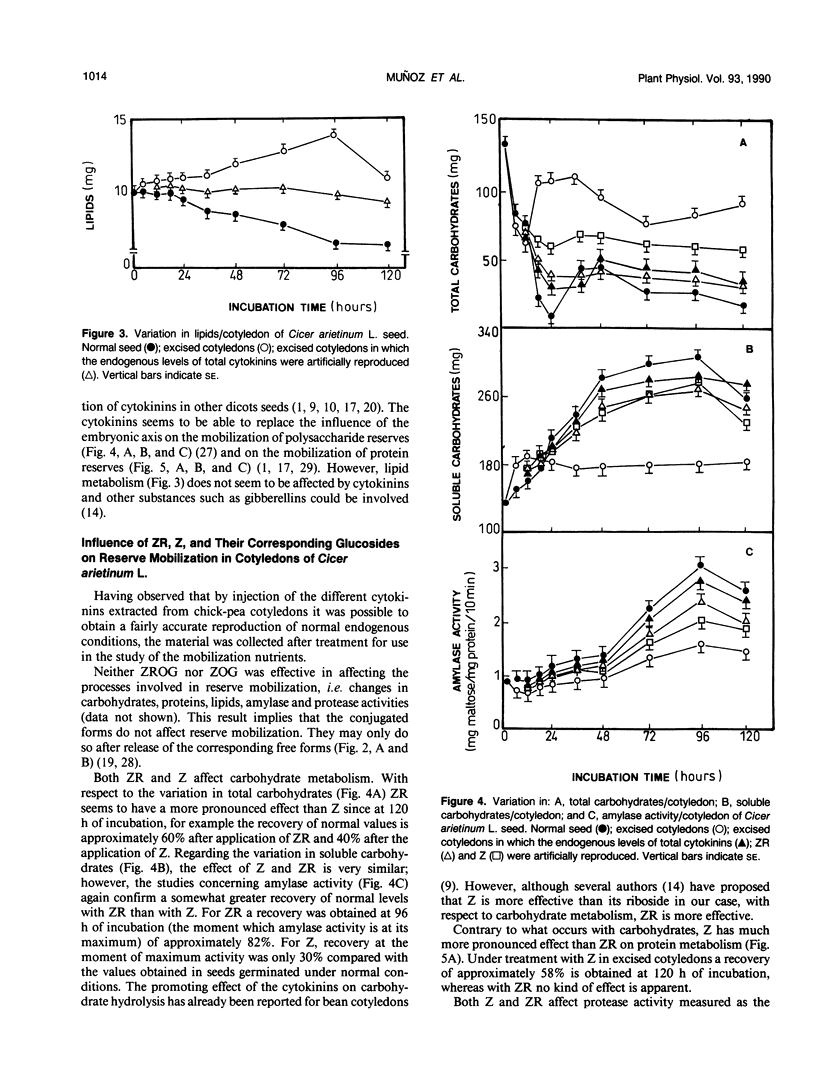
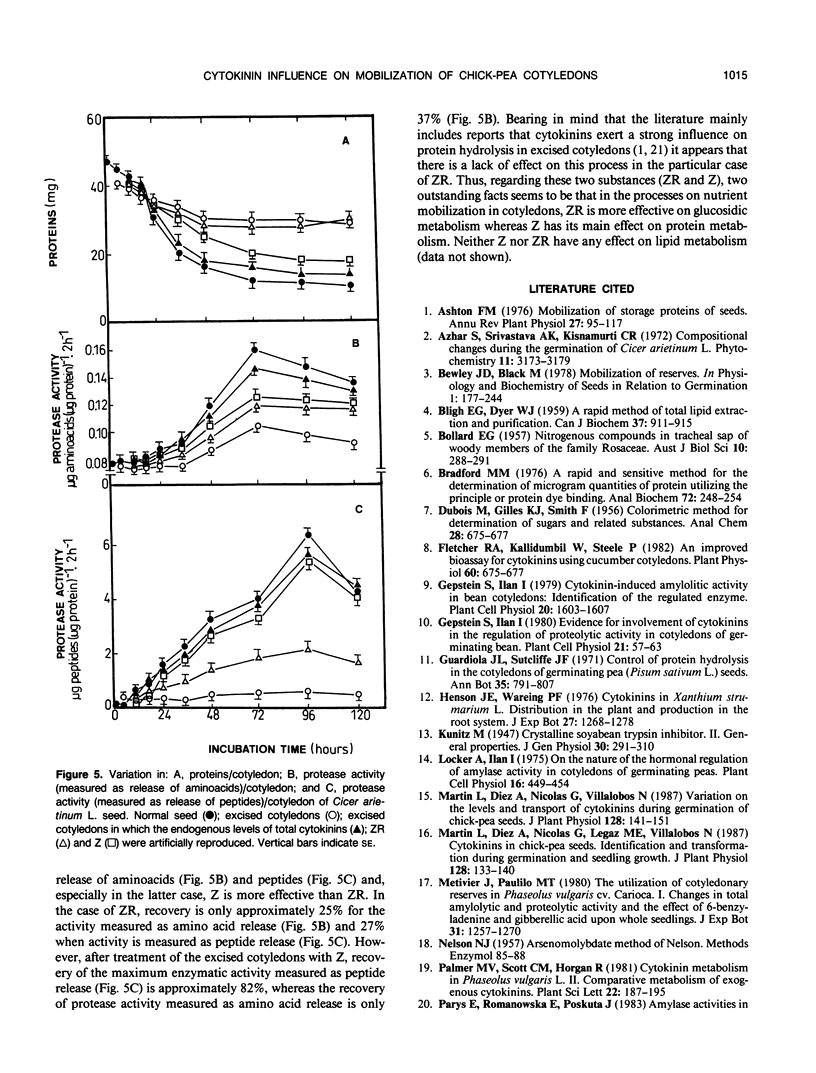
Abstract
The embryonic axis plays an essential role in the mobilization of the main reserves of the cotyledons of seeds of Cicer arietinum L. cv Castellana. This control by the axis of the metabolism of the storage products of the cotyledons largely takes place through the cytokinins, which are transported from the embryonic axis to the cotyledons where the mobilization of reserves begins. The principal regulatory role of the endogenous cytokinins concerns the metabolism of carbohydrates and proteins; there is less influence on lipid metabolism. However, each cytokinin seems to have a different role in the mobilization processes. The glucosides, glucosyl zeatin riboside, and glucosyl zeatin act only as storage forms of the hormones. Zeatin riboside affects mainly the mobilization of carbohydrates and has less effect on protein mobilization. Zeatin regulates both the mobilization of carbohydrates and that of proteins and is more marked in the latter case.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Fletcher R. A., Kallidumbil V., Steele P. An improved bioassay for cytokinins using cucumber cotyledons. Plant Physiol. 1982 Mar;69(3):675–677. doi: 10.1104/pp.69.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner D., Ashton F. M. Hormonal control of proteinase activity in squash cotyledons. Plant Physiol. 1967 Jun;42(6):791–796. doi: 10.1104/pp.42.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Varner J. E., Balce L. V., Huang R. C. Senescence of Cotyledons of Germinating Peas. Influence of Axis Tissue. Plant Physiol. 1963 Jan;38(1):89–92. doi: 10.1104/pp.38.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yomo H., Varner J. E. Control of the formation of amylases and proteases in the cotyledons of germinating peas. Plant Physiol. 1973 Apr;51(4):708–713. doi: 10.1104/pp.51.4.708. [DOI] [PMC free article] [PubMed] [Google Scholar]


