Abstract
Aim
One of the main reasons for the death due to snake bites is the non-availability of antivenoms in the regions where they are needed. The use of medicinal plants and plant-based natural products as an alternative to antivenom will become a milestone in snake bite envenomation. The present study investigates the in vitro antivenom properties of Cyanthillium cinereum root extracts.
Materials and methods
The C. cinereum root's aqueous extract was prepared by the Soxhlet extraction method, and phytochemical screening was performed to detect the presence of various bioactive compounds. Thin-layer chromatography (TLC) and gas chromatography–mass spectrometry (GC–MS) analysis were performed for the detection and identification of phytochemical constituents. In this study, an in vitro model is used to assess the antivenom capability of aqueous extract. Venom toxicity and neutralization assays were as follows: An in vitro pharmacological evaluation was performed by direct hemolysis assay, indirect hemolytic assay, proteolytic activity, neutralization of procoagulant activity, and gelatin liquefaction method.
Results
Qualitative analysis of phytochemicals by the standard method showed the presence of various phytochemical constituents. Also, GC–MS analysis showed the presence of three major compounds that possess antivenom activity from the obtained 60 bioactive compounds, and their chemical structures were also determined. Venom protein profiling was performed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis. The plant extract was able to neutralize the Naja naja (N. naja) and Daboia russelii (D. russelii) venom induced hemolysis and it was reduced below 50 and 40%, respectively and the extract was also able to reduce the hemolytic halo produced by venoms. Procoagulant activity and gelatin liquefaction assay showed that venom-induced clotting was neutralized by increasing the root extract concentration sufficiently.
Conclusion
The aqueous extract of the root of C. cinereum showed potent in vitro venom-neutralizing activity, and it can be used as a formidable therapeutic agent against N. naja and D. russelii envenomation.
How to cite this article
Suji S, Dinesh MD, Keerthi KU, Anagha KP, Arya J, Anju KV. Evaluation of Neutralization Potential of Naja naja and Daboia russelii Snake Venom by Root Extract of Cyanthillium cinereum. Indian J Crit Care Med 2023;27(11):821–829.
Keywords: Antivenom activity, Cyanthillium cinereum, Daboia russelii, Envenomation, Naja naja, Root extract, Single ingredient
Highlights
By using in vitro techniques, the anti-venom activity of Cyanthillium cinereum plant root extracts against the venom of Naja naja (N. naja) and Daboia russelii (D. russelii) was examined.
The phytochemical screening and gas chromatography–mass spectrometry (GC–MS)/mass spectrometry (MS) analysis were used to infer the C. cinereum plant root extract's compound richness in aqueous extracts.
Findings contain substances that can be used in traditional wisdom medication in the form of a single ingredient or in combination for the incident of snake envenomation.
Introduction
A snakebite is a severe, time-sensitive medical emergency. Due to heavy rains and humid weather, it is a public health issue that primarily affects the rural population.1,2 In addition to having some of the densest agrarian communities in the world, the Southeast Asia Region is a biodiversity hotspot for poisonous snakes. Nearly 70% of the predicted worldwide mortality from snakebite is attributed to snakebites in the area. According to studies just from India, there are between 0.77 and 1.24 million cases of snakebite envenoming every year, resulting in 58,000 fatalities.3
Geographically, snakebites have the most effects in tropical and subtropical areas, with India having the most cases of them. There are four commonly found poisonous snakes in India—common cobra, common krait, Russell's viper, and saw-scaled viper known as the “Big Four.”4
Snake exposure increased as a result of traditional farming practices. Furthermore, improper or ineffective treatment and consequences result from incorrect or inaccurate identification of the snake species.5 Limited access to relevant services, the impact of health-seeking behavior on obtaining such services, and a lack of effective antivenom are all factors in this.6,7
Several snake species have varied envenomation progression patterns. Many snakes deliver highly lethal venom through modified salivary glands. Venom is transferred from glands to fangs via a duct during the envenomation process, where it eventually reaches the prey, killing it. Also, certain snakes strike their prey without injecting poison (dry bite).8,9 Hysteria, which causes an elevated heartbeat, emesis, light-headedness, tachycardia, hypotension, vertigo, and sticky and perspiring skin, are the characteristic symptoms of snake envenomation.10
Hemotoxic, neurotoxic, and cytotoxic are the three major subtypes of the pharmacological effects of snake venom.11 Phospholipase A2 (PLA2), snake venom metalloproteases, snake venom serine proteases, and three-finger toxins (3FTXs) are the main toxins involved in these actions, which either independently or jointly lead to the varied pharmacological reactions found in snakebite patients.12–16
The rapid delivery of bivalent or polyvalent antivenom is the most efficient and widely accepted treatment for a poisonous snakebite.17,18 The currently available polyvalent ASV is expensive and hard to come by, especially in high-risk locations. However, it is effective against bites from common neurotoxic and hemotoxic snakes.19
Their usage in clinical settings is constrained by allergic reactions involved with the antibodies in polyvalent antivenoms. A number of issues, including their high cost, wide range of side effects, stringent storage requirements, lack of specificity, and risk of immunological reactions, have limited the use of antivenoms.9
However, antivenom therapy has a number of negative consequences for the treated patient, including pyrogenic responses, anaphylaxis, and serum sickness.20,21 For treating snakebite patients, phytotherapy may be more practical and less expensive.22 By chewing leaves or bark, drinking plant extracts, injecting them, or applying them topically to the affected areas, various plant species have historically been used to detoxify snake venom. However, right now, to treat snake envenomation, traditional healers only use pure plant remedies.23
Gunjan Guha et al. reported the activity of C. cinereum as an antidote against scorpion stings and snake venom using root extracts.24 A traditional plant of high medicinal benefit in several traditional uses in various countries, including the ayurvedic system of medicine, C. cinereum, also known as Vernonia cinerea or commonly known as little ironweed, is one of these. We, therefore, set out to assess C. cinereum's antivenom ability utilizing root extract against the venoms of N. naja and D. russelii.
Materials and Methods
Collection and Preparation of Extract
Based on oral literature collected from herbal drug practitioners, C. cinereum, sometimes referred to as little ironweed, is a medicinal plant collected from the herbal gardens of our college. The plant was authentically identified from Kottakkal Arya Vaidya Sala, Kanjikode, Palakkad, Kerala, India. The root extracts of C. cinereum was prepared by Soxhlet method using water as a solvent.
Phytochemical Analysis
According to a conventional qualitative test that has been previously described by many researchers, the root extract was subjected to phytochemical analysis. The sample extract was screened in search of bioactive substances such as alkaloids,25 saponins,26 tannins,27 anthraquinone,28 flavonoids by FeCl2 test,29 phenol (alkaline reagent), steroids and terpenoids (Liebermann–Burchard's reaction), cholesterol (Salkowski test), phytosterols,30 proteins by Millon's test,31 amino acids by Ninhydrin test,32 sugars by Anthrone method,33 reducing sugar (Benedict's test) and glycosides (Keller-Kiliani test).
Separation of Plant Components using Thin-layer Chromatography (TLC)
Silica gel (Merc-105553) was used to create a thin layer for the TLC plate and kept in a hot air oven at 110°C for 45 minutes for the activation of silica gel. The plates were developed in a chromatographic tank using a solvent system including methanol and in (6:4) ratio. Following TLC spots were detected after spraying with FeCl3 reagent.34
Determination of Specific Phytocompounds of C. cinereum by Gas Chromatography–Mass Spectrometry/Mass Spectrometry
The active chemicals found in the C. cinereum root extract were further identified using GC–MS/mass spectrometry (MS) analysis. Chromatographic separation was carried out at the Central Instrumentation Laboratories, College of Veterinary and Animal Sciences, Mannuthy, Thrissur, Kerala, India using the TSQ GC–MS/MS System. The essential chemical components were identified by comparing their mass spectra with reference compounds’ mass spectra from the mass spectral collection (NIST 147) of the National Institute of Standards and Technology. As a percentage of the total peak areas, the relative amounts of the various components were expressed.
Protein Profiling and Estimation of the Total Protein Content of Venoms
The Irula Snake Catcher's Industrial Cooperative Society, Chennai, Tamil Nadu, India provided the freeze-dried venom powders of D. russelii and N. naja, which were kept at 4°C. SDS–PAGE was used to evaluate the protein profile of snake venom, and its purity was compared with known molecular weight markers (low- and mid-range market, Genei Pvt. Ltd, Bengaluru, Karnataka, India).35 Physiological saline and lyophilized venom at a concentration of 1 mg/mL made up the stock solution. Folin and Ciocalteau chemicals were used to calculate the total protein concentration using the Lowry method.
Venom Toxicity and Neutralization Assays: An In Vitro Pharmacological Assessment
Direct Hemolysis Assay
Red blood cell (RBC) was used in an in vitro study to examine the hemolytic activity of the venoms and root extracts of N. naja and D. russelii. 5 mL of citrated blood was centrifuged at 900 rpm for 10 minutes. After pouring off the supernatant, a physiological salt solution was used to wash the pellet twice. A 5 mL physiological saline and 0.5 mL RBC mixture were used as controls. Furthermore, 100% hemolysis was accomplished with distilled water and washed RBC in the same ratio. Also, 5 mL of venom extract and 0.5 mL of washed RBC made up the test sample. The tubes were centrifuged for 20 minutes at 2000 rpm after spending an hour at 37°C in a thermostat. To evaluate the optical density of the supernatant against water, a spectrophotometer was set at a wavelength of 540 nm and measured.
The following formula was used to calculate hemolysis:

Indirect Hemolysis Assay
Using an agarose–erythrocyte–egg yolk gel plate technique, the PLA2 activity was determined.36 Also, 10 mL of venoms from N. naja and D. russelii were added into 3 mm wells of agarose gels along with 1.2% sheep erythrocytes, 1.2% egg yolk as a source of lecithin, and 10 mM CaCl2 15 µL of saline were present in the control wells. After incubating the slides for a night at 37°C, the diameters of the hemolytic haloes were measured. The venom concentration that created a hemolytic halo with a diameter of 11 or 10 mm, respectively, is the minimum indirect hemolytic dose (MIHD).
By combining a fixed amount of venom (µg) with a variable amount of plant root extract (µL), and incubating the mixture for 30 minutes at 37°C, it was possible to determine how effectively C. cinereum root extracts neutralized the phospholipase activity (PLA2). A 10 µL aliquot of each combination was placed in a well created on a slide that had gels in it. The control sample only contains venom. At 37°C, the plates were incubated for 20 hours. When compared to the effect caused by venom alone, the diameter of the hemolytic halo is reduced by 50% through neutralization, which is measured as the ratio of mg plant extract/mg venom.36
Proteolytic Activity
The plates were made using skim milk agar (1%).37 The venom of N. naja and D. russelii was added to each of the four agar wells on a plate, which was then incubated at 37°C for 24 hours. As a control, 50 µL of phosphate buffered saline (PBS) was used. A preincubation with different quantities of plant root extracts with the same concentration of venom at 37°C for 1 hour was done for the neutralization assay. These samples were then put on agar plates, which were then incubated for 24 hours at 37°C. The milk agar plate's casein hydrolysis zone was measured. The amount of plant extract to venom that could reduce the diameter of the zone of hydrolysis by 50% was defined as neutralization as compared to the impact brought on by venom alone.36
Neutralization of Procoagulant Activity
A modified version of Theakston and Reid's approach, developed by Gene was used to measure the procoagulant activity.38,39 The C. cinereum root extracts were diluted in various ratios with a consistent amount of venom, and after that, the mixture was incubated at 37°C for 30 minutes. The clotting times were then recorded after 0.2 mL of citrated plasma samples were combined with 0.1 mL of a mixture containing two minimal coagulant doses of venom. Incubation of the plasma with either extracts or with the venom alone was done in control tubes. Neutralization was measured as the effective dose (ED) by comparing the concentration of mg extracts with the concentration of venom at which clotting time triples compared to the concentration of two MCDs of venom alone in plasma incubated.
Neutralization Assay using Gelatin-liquefaction Method
A constant volume of venom and gelatin were added to the variable dilutions of root extracts, and then they were incubated at 37°C for 30 minutes. Gelatin was only incubated with venom in control tubes. The liquefaction of gelatin after treatment with varying amounts of plant extracts was an indication of neutralization.
Results
The C. cinereum root was finely powdered and carried on with soxhlation against distilled water as a solvent. A rotary evaporator was used to evaporate the plant root extract, and a freeze dryer was used to further lyophilize the resulting sample. The C. cinereum's root extracts were subjected to phytochemical analysis, which revealed the presence of active secondary metabolites like saponin, tannin, phenols, flavonoids, and terpenoids. Also, it shows negative results for anthraquinones and alkaloids (Table 1). The C. cinereum root extracts were subjected to TLC using methanol and water (6:4) solvent systems. After spraying with the FeCl3 reagent, spots were found using TLC (Fig. 1).33
Table 1.
Secondary metabolite phytochemical screening and its conclusion
| Test | Result |
|---|---|
| Saponin | + |
| Tannins | + |
| Anthraquinone | − |
| Flavonoids | + |
| Phenols | + |
| Alkaloids | − |
| Terpenoids | + |
Symbol “+” indicates the presence of phytochemicals; Symbol “−” indicates the absence of phytochemicals
Fig. 1.
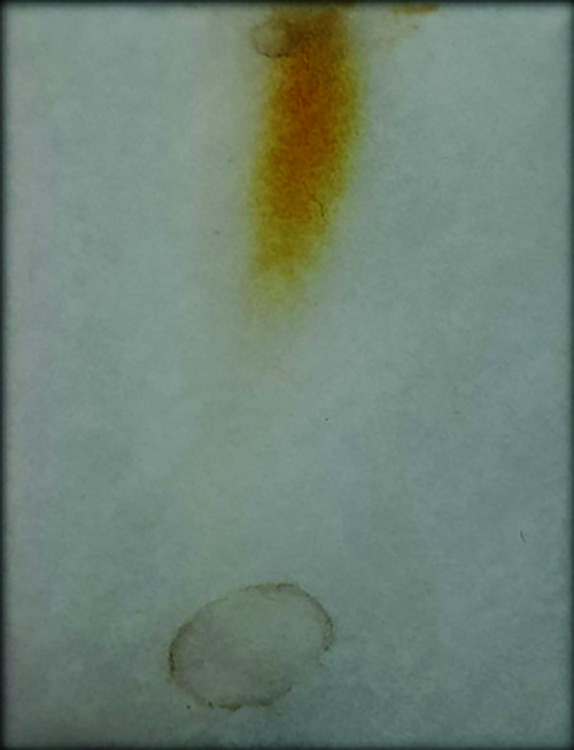
Thin layer chromatography of C. cinereum root extract
The Lowry method was used to assess the protein concentration in the lyophilized snake venoms.40 Also, 840 mg/mL and 222.2 mg/mL of total protein were discovered in the venom of the N. naja and D. russelii, respectively. The effects of the many proteinaceous components found in snake venom combined to make it poisonous.
The protein profile of both venoms was analyzed by SDS–PAGE. Their banding pattern was visualized by Coomassie Brilliant Blue staining. A group of protein bands was observed for both venoms (Fig. 2).
Fig. 2.
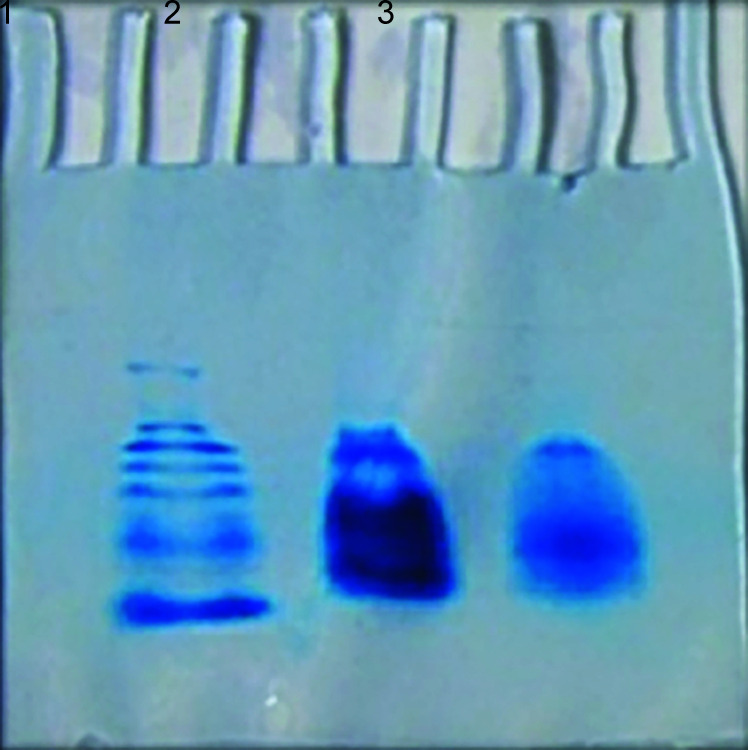
The SDS–PAGE protein profile of snake venom. 1, Marker; 2 Russell's viper; 3, Indian cobra
A study on the direct hemolysis of N. naja and D. russelii venom utilizing sheep RBCs showed that the RBCs have been lysed by snake venom. Also, N. naja venom showed 85% and D. russelii venom showed 90.5% of hemolysis. By using plant root extract of C. cinereum, the hemolysis brought on by the venoms was reduced to less than 50 and 40%, respectively; (Fig. 3) shows the neutralizing ability of the root extract on the experimental venoms.
Fig. 3.
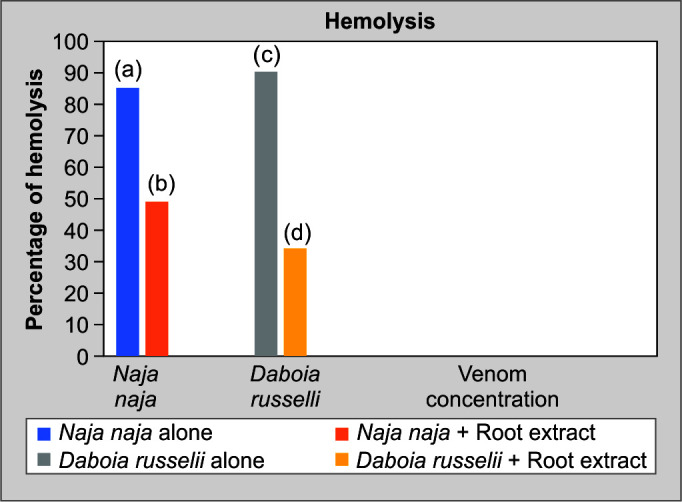
Direct hemolysis activity of N. naja and D. russelii venoms (neutralization assay)
A 10 μg of PLA2 activity hemolytic halo, which is regarded as “1 Unit,” can be produced by the venoms. Plant extracts inhibited PLA2-dependent hemolysis of RBCs induced by snake venom in a dose-dependent manner; (Fig. 4) demonstrates the inhibition of both venom's phospholipase enzymes by plant extract by measuring the zone's size.
Fig. 4.
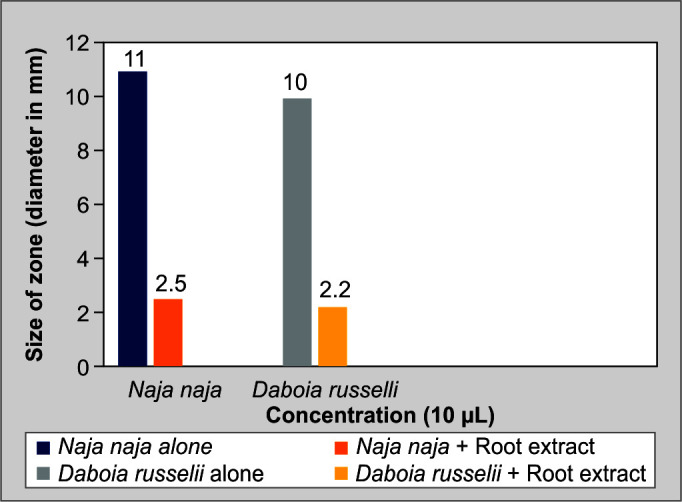
Indirect hemolysis assay: PLA2
The skim milk agar plate method was used to assess the proteolytic activity of snake venom. N. naja and D. russelii venoms produced hydrolysis zones with diameters of 1.5 and 1.1 cm, respectively. To conduct neutralization experiments, different amounts of plant extracts were first incubated with venom at defined doses. When proteolytic activity was carried out, it became clear that plant extracts had a greater capacity to reduce the diameter of the zone of hydrolysis than venom alone did. The ratio of plant extract to venom was used to quantify this neutralization. The hydrolysis zone of the venom can be reduced by plant extracts; Figures 5 and 6 exhibit the capacity of the plant extract to neutralize the proteolytic enzyme in the venom samples.
Fig. 5.
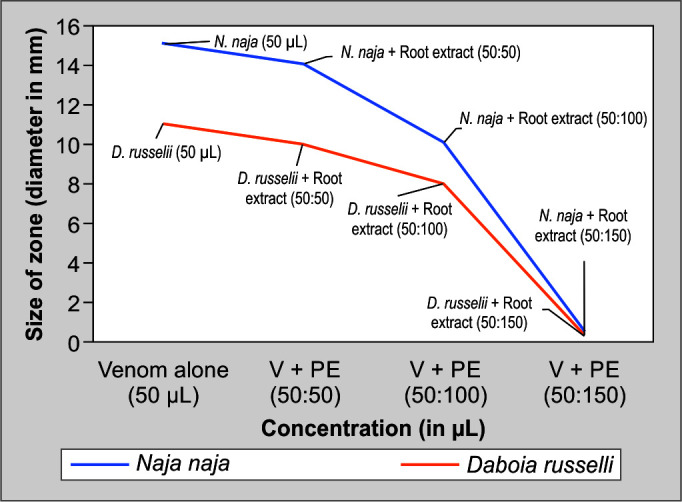
Proteolytic activity of N. naja and D. russelii
Fig. 6.
Proteolytic activity of snake venoms and its neutralization assay
At 37°C, different concentrations of venom were mixed with 0.1 mL of citrated human plasma. The minimum coagulant dose was determined to be the venom concentration that induces plasma to clot in 60 seconds. By sufficiently raising the dosage of antivenoms, venom-induced coagulation was neutralized. One minimal coagulant dose of venom and various extract amounts were mixed in 0.1 mL of human citrated plasma. Clot formation was not present, demonstrating the plant extracts’ potential to serve as neutralizers. Rapid clotting produced by high venom concentrations requires extremely high antivenom concentrations to inhibit it. Extracts from the roots of C. cinereum are efficient at reducing the coagulant effects brought on by snake venom; (Fig. 7) shows the venom-induced clotting was reduced by the neutralizing effect of root extract.
Fig. 7.
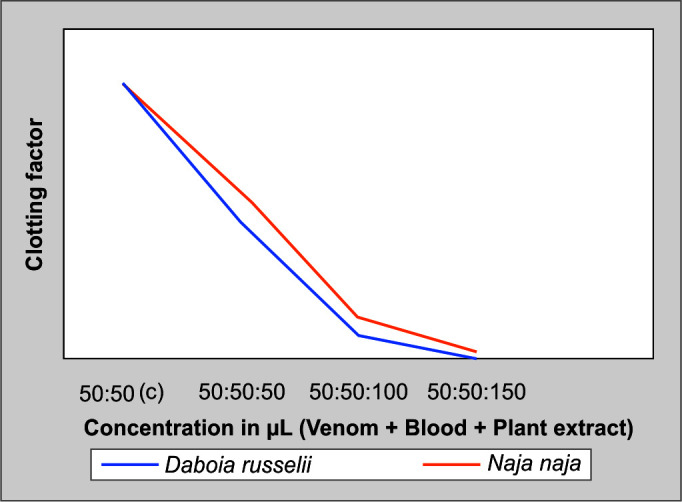
Procoagulant activity of N. naja and D. russelii venom and its neutralization assay
In the tube holding 50 µL of venom and gelatin, different concentrations of root extracts were dissolved. One minimum coagulant dose of venom and varied extract amounts were dissolved in 50 µL of gelatin. The lack of clot formation demonstrates how plant extracts have a neutralizing effect; (Fig. 8) exhibits how the inhibitory characteristic of root extracts of C. cinereum is used to neutralize the coagulant activity induced by snake venoms by gelatin liquefaction method.
Fig. 8.
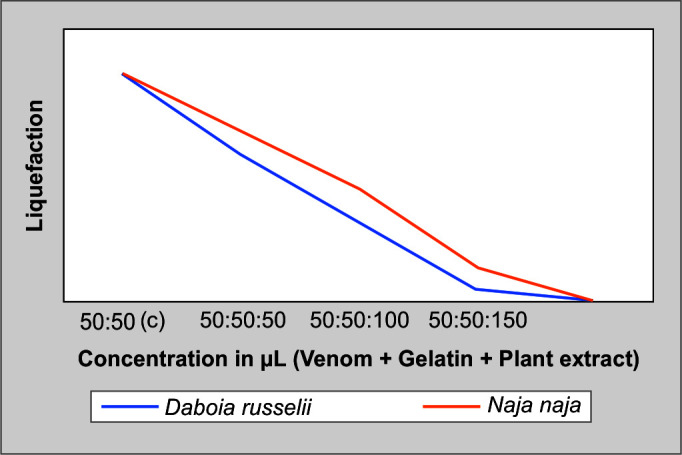
Gelatin liquefaction assay of N. naja and D. russelii
The C. cinereum root extracts have shown the existence of medicinally active ingredients. The bioactive phytochemical components in the aqueous extract of the root have been identified using GC–MS/MS analysis. Phytochemical substances are recognized based on the peak area, molecular weight, and molecular formula, but some of the GCMS peaks remain unidentified. The major 12 peaks, with each peak having 3–5 possible compounds, have been identified and illustrated in Table 2 and in Figure 9 that shows the GC–MS analysis of aqueous root extract of C. cinereum.
Table 2.
The major compounds in the aqueous root extract of C. cinereum
| RT | S.No. | IUPAC name | Chemical formula |
|---|---|---|---|
| 4.57 | 1 | à-D-Glucopyranoside, O-à-D-glucopyranosyl-(1. fwdarw.3)-á-D-fructofuranosyl |
C18H32O16 |
| 4.57 | 2 | 9-Hexadecenoic acid | C16H30O2 |
| 17.49 | 3 | n-Hexadecanoic acid | C16H32O2 |
| 18.57 | 4 | Octasiloxane, 1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-hexadecamethyl- | C16H48O7Si8 |
IUPAC, international Union of Pure and Applied Chemistry; RT, retention time. In vitro antivenom studies of the aqueous root extract of C. cinereum show considerable results against inhibition of coagulant and neutralization of phospholipase activities caused by cobra venom and Russell's viper
Fig. 9.
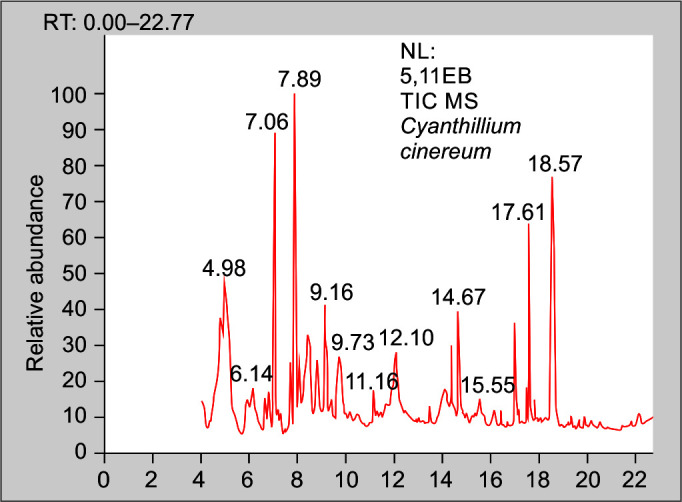
Chromatogram 1: GC–MS analysis of aqueous root extract of C. cinereum
In-vitro antivenom studies of the aqueous root extract of C. cinereum show considerable results against inhibition of coagulant and neutralization of PLA2 activities caused by cobra venom and Russell's viper. According to the GC–MS/MS data, four major compounds were identified, representing the antivenom properties of the total identified compounds. The detected compounds were mainly composed of esters, trisaccharides, fatty acids, and others.
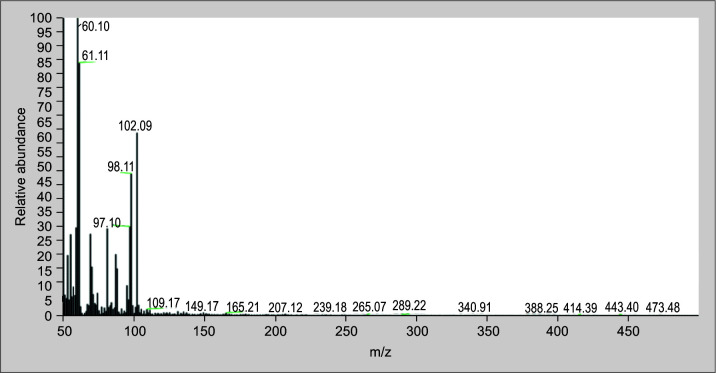
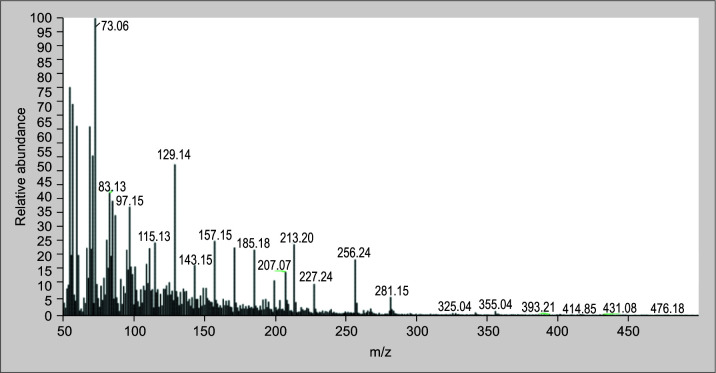
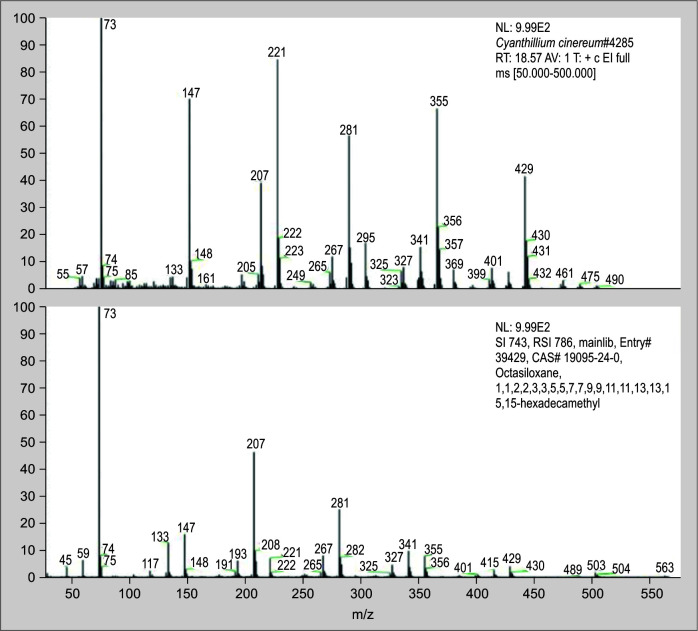
The root extract of C. cinereum contains a number of known substances, such as 9-hexadecenoic acid, n-hexadecenoic acid octasiloxane,1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-hexadecamethyl- (Figs 10 to 12 illustrate the mass spectra of identified compounds from aqueous extract of C. cinereum root) which inhibits coagulant activity, inhibits hemolytic activity, and effectively neutralizes PLA2.
Fig. 10.
Spectrum of 9-hexadecenoic acid from C. cinereum aqueous root extract identified by GC–MS analysis
Discussion
In consideration of the fact that snakebite envenoming primarily affects rural, underprivileged populations in the tropics, the World Health Organization recognized snakebite as a major neglected tropical disease (NTD). Along with regional swelling, blistering, and tissue necrosis (cytotoxicity) near the bite site, a snakebite can also result in neuromuscular paralysis (neurotoxicity), bleeding, and coagulopathy (hemotoxicity). Many scientists have concentrated their study on the production of antisnake venom compounds from various botanical sources.
Traditional healers generally use plant extracts, especially in tropical areas, to cure snakebite envenomation from abundant herbal sources.41 Identifying the precise phytoconstituent responsible for a particular antisnake venom activity is the main challenge in the development of antivenom drugs made from plants.
In our investigation here, an attempt is made to reveal the traditional antivenom property of C. cinereum root extracts against N. naja and D. russelii venoms using in-vitro techniques in order to prove the traditional applications and their pharmacological evidence.
For the purpose of the study, C. cinereum was obtained from the herbal garden of our institution in Coimbatore, and it was officially authenticated from Kottakkal Arya Vaidya Sala, Kanjikode, Palakkad, Kerala, India. To prepare the plant root extract, distilled water was employed as the solvent system. The dried sample was thoroughly mixed with distilled water, and samples were analyzed for qualitative determination by phytochemical screening and GC–MS/MS analysis. The phytochemical analysis revealed the presence of active secondary metabolites like saponin, tannin, flavonoids, phenols, and terpenoids. It shows few negative results, indicating the absence of alkaloids and anthraquinones. To identify and understand the chemical components that make up C. cinereum root extracts, were subjected to TLC using a methanol and water (6:4) solvent system.
According to the discussions and findings from the ultraviolet (UV) trans-illuminator observations, the movement of spots was observed at a definite distance in the extracts. For the purpose of this present study, snake venom samples from N. naja and D. russelii were bought in lyophilized form from Irula Snake Catchers Cooperative Society Limited, Chennai, Tamil Nadu, India. For both venom samples, the total protein was calculated using the Lowry method. 840 mg/mL and 222.2 mg/mL were found to be the venom concentrations of N. naja and D. russelii, respectively. The protein profile of both the snake venoms was analyzed by SDS–PAGE.
For the in vitro neutralization assay, both direct and indirect experiments were performed. We performed direct hemolysis using RBCs and showed that both snake venoms were effective at lysing the RBCs. The PLA2 measurements showed that 10 μg of the venoms of N. naja and D. russelii could produce hemolytic haloes that were 11 and 10 mm in size, respectively.
The venomous zone of hydrolysis can be lessened by proteolytic activity. Procoagulant activity illustrates the impossibility of clot formation and the capacity of plant extracts to inhibit it. The lack of clot formation and the neutralizing capacity of plant extracts are shown using gelatin liquefaction experiments.
Our in vitro neutralization tests against the venoms of N. naja and D. russelii proved that C. cinereum plant had antivenom properties. The root seems to act both directly and indirectly against both venoms. The results of our primary study indicate that C. cinereum's antivenom-producing abilities are brought about by bioactive phytochemicals, including fatty acids and ester compounds. The C. cinereum's aqueous extract contained 60 bioactive phytochemical components that were identified using GC–MS/MS analysis. The major 12 peaks, with each peak having 3–5 possible compounds, have been identified in C. cinereum root extracts. The chemical structure and structural details of each active component in C. cinereum's root extract have been reviewed in detail.
Among the compounds identified, 9-hexadecenoic acid, (Fig. 10) which is a monounsaturated fatty acid found in C. cinereum's root, helps in the design of specific inhibitors of PLA2 enzymes. Also, n-hexadecenoic acid (Fig. 11) is also a saturated fatty acid compound, and its enzyme kinetics proved that it inhibits PLA2 in a competitive manner.41 According to Gnanaselvan and Sivaraman, the hemolytic action of the cardiotoxins in the N. naja is inhibited by the molecule octasiloxane, a 1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-hexadecamethyl-an ester (Fig. 12).42 The root of C. cinereum has antivenom characteristics because of these chemical constituents.
Fig. 11.
Spectrum of n-hexadecenoic acid from C. cinereum aqueous root extract identified by GC–MS analysis
Fig. 12.
Spectrum of octasiloxane, 1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-hexadecamethyl- from C. cinereum aqueous root extract identified by GC–MS analysis
Orcid
Suji S https://orcid.org/0009-0000-3520-8846
Dinesh MD https://orcid.org/0000-0003-1466-6922
Keerthi KU https://orcid.org/0009-0008-5062-3042
Anagha KP https://orcid.org/0009-0002-0519-533X
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Majumder D, Sinha A, Bhattacharya SK, Ram R, Dasgupta U, Ram A. Epidemiological profile of snake bite in south 24 Parganas district of West Bengal with focus on underreporting of snake bite deaths. Indian journal of public health. 2014;58(1):17–21. doi: 10.4103/0019-557X.128158. [DOI] [PubMed] [Google Scholar]
- 2.Mohapatra B, Warrell DA, Suraweera W, Bhatia P, Dhingra N, Jotkar RM, et al. Snakebite mortality in India: A nationally representative mortality survey. PLoS Negl Trop Dis. 2011;5(4):e1018. doi: 10.1371/journal.pntd.0001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharati K. Snakebite management in India: Challenges remain. EFI Bulletin. 2023;4(1):32–34. doi: 10.56450/EFIB.2023.v3i01.008. [DOI] [Google Scholar]
- 4.Bawaskar HS. Snake venoms and antivenoms: Critical supply issues. J Assoc Physicians India. 2004;52:11–13. 15633710 [PubMed] [Google Scholar]
- 5.Saravu K, Somavarapu V, Shastry AB, Kumar R. Clinical profile, species-specific severity grading, and outcome determinants of snake envenomation: An Indian tertiary care hospital-based prospective study. Indian J Crit Care Med. 2012;16(4):187–192. doi: 10.4103/0972-5229.106499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martín G, Erinjery J, Gumbs R, Somaweera R, Ediriweera D, Diggle PJ, et al. Integrating snake distribution, abundance and expert‐derived behavioural traits predicts snakebite risk. J Appl Ecol. 2022;59(2):611–623. doi: 10.1111/1365-2664.14081. [DOI] [Google Scholar]
- 7.Chippaux JP. Estimating the global burden of snakebite can help to improve management. PLoS Med. 2008;5(11):e221. doi: 10.1371/journal.pmed.0050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rita P, Animesh DK, Aninda M, Benoy GK, Sandip H, Datta K. Snake bite, snake venom, anti-venom and herbal antidote. A review. Int J Res Ayurveda Pharm. 2011;2(4):1060–1067. [Google Scholar]
- 9.Félix–Silva J, Silva–Junior AA, Zucolotto SM, Fernandes–Pedrosa MD. Medicinal plants for the treatment of local tissue damage induced by snake venoms: an overview from traditional use to pharmacological evidence. Evidence-Based Complementary and Alternative Medicine. 2017;2017:5748256. doi: 10.1155/2017/5748256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutiérrez JM, Theakston RD, Warrell DA. Confronting the neglected problem of snake bite envenoming: The need for a global partnership. PLoS Med. 2006;3(6):e150. doi: 10.1371/journal.pmed.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancet T. Snake bite: Time to stop the neglect. The Lancet. 2010;375(9708):2. doi: 10.1016/S0140-6736(09)62168-1. [DOI] [PubMed] [Google Scholar]
- 12.Fry BG, Winkel KD, Wickramaratna JC, Hodgson WC, Wüster W. Effectiveness of snake antivenom: Species and regional venom variation and its clinical impact. J Toxicol Toxin Rev. 2003;22(1):23–34. doi: 10.1081/TXR-120019018. [DOI] [Google Scholar]
- 13.Lynch VJ. Inventing an arsenal: Adaptive evolution and neofunctionalization of snake venom phospholipase A2 genes. BMC Evol Biol. 2007;7(1):2. doi: 10.1186/1471-2148-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunagar K, Fry BG, Jackson TN, Casewell NR, Undheim EA, Vidal N, et al. Molecular evolution of vertebrate neurotrophins: Co-option of the highly conserved nerve growth factor gene into the advanced snake venom arsenalf. PloS One. 2013;8(11):e81827. doi: 10.1371/journal.pone.0081827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris JB, Scott–Davey T. Secreted phospholipases A2 of snake venoms: effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins. 2013;5(12):2533–2571. doi: 10.3390/toxins5122533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsetlin VI. Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: Pharmacological tools and endogenous modulators. Trends Pharmacol Sci. 2015;36(2):109–123. doi: 10.1016/j.tips.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee AK, Mackessy SP. Prevention and improvement of clinical management of snakebite in Southern Asian countries: A proposed road map. Toxicon. 2021;200:140–152. doi: 10.1016/j.toxicon.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Patra A, Banerjee D, Dasgupta S, Mukherjee AK. The in vitro laboratory tests and mass spectrometry-assisted quality assessment of commercial polyvalent antivenom raised against the ‘Big Four’ venomous snakes of India. Toxicon. 2021;192:15–31. doi: 10.1016/j.toxicon.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Gadwalkar SR, Kumar NS, Kushal DP, Shyamala G, Mohammad MZ, Vishwanatha H. Judicious use of antisnake venom in the present period of scarcity. Indian J Crit Care Med. 2014;18(11):722. doi: 10.4103/0972-5229.144014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makhija IK, Khamar D. Anti-snake venom properties of medicinal plants. Der Pharmacia Lettre. 2010;2(5):399–411. doi: 10.1590/s2175-97902022e191124. [DOI] [Google Scholar]
- 21.Mukherjee AK. Green medicine as a harmonizing tool to antivenom therapy for the clinical management of snakebite: The road ahead. Indian J Med Res. 2012;136(1):10–12. 22885258 [PMC free article] [PubMed] [Google Scholar]
- 22.Adeyemi S, Larayetan R, Onoja AD, Ajayi A, Yahaya A, Ogunmola OO, et al. Anti-hemorrhagic activity of ethanol extract of Moringa oleifera leaf on envenomed albino rats. Scientific African. 2021;12:e00742. doi: 10.1016/j.sciaf.2021.e00742. [DOI] [Google Scholar]
- 23.Butt MA, Ahmad M, Fatima A, Sultana S, Zafar M, Yaseen G, et al. Ethnomedicinal uses of plants for the treatment of snake and scorpion bite in Northern Pakistan. J Ethnopharmacol. 2015;168:164–1681. doi: 10.1016/j.jep.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Guha G, Rajkumar V, Mathew L, Kumar RA. The antioxidant and DNA protection potential of Indian tribal medicinal plants. Turkish J Biol. 2011;35(2):233–242. doi: 10.3906/biy-0906-64. [DOI] [Google Scholar]
- 25.Evans WC. 14th edition. Singapore: Harcourt Brace and Company; 1997. Trease and Evans Pharmacognosy, [Google Scholar]
- 26.Qiu SX, Cordell GA, Kumar BR, Rao YN, Ramesh M, Kokate C, et al. Bisdesmosidic pregnane glycosides from Caralluma lasiantha. Phytochemistry. 1999;50(3):485–491. doi: 10.1016/s0031-9422(98)00569-x. [DOI] [Google Scholar]
- 27.Mace ME. Histochemical localization of phenols in healthy and diseased banana roots. Physiologia Plantarum. 1963;16(4):915–925. doi: 10.1111/j.1399-3054.1963.tb08367.x. [DOI] [Google Scholar]
- 28.Evans WC, Trease GE. 15th edition. London: Saunders; 2002. Trease and Evans Pharmacognosy, [Google Scholar]
- 29.Sofowora A. Recent trends in research into African medicinal plants. J Ethnopharmacol. 1993;38(2–3):209–214. doi: 10.1016/0378-8741(93)90017-y. [DOI] [PubMed] [Google Scholar]
- 30.Upadhyaya SK, Singh V. Phytochemical evaluation of Cassia obtusifolia L. and Cassia tora L. Proc Indian Acad Sci Plant Sci. 1986;96(4):321–326. doi: 10.1007/bf03053254. [DOI] [Google Scholar]
- 31.Rasch E, Swift H. Microphotometric analysis of the cytochemical Millon reaction. J Histochem Cytochem. 1960;8(1):4–17. doi: 10.1177/8.1.4. [DOI] [PubMed] [Google Scholar]
- 32.Yasuma A, Ichikawa T. A new Histochemical staining method for protein. J Lab Clin Med. 1953;41(2):296–299. 13035263 [PubMed] [Google Scholar]
- 33.Yemm EW, Willis A. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954;57(3):508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banu HR, Nagarajan N. TLC and HPTLC fingerprinting of leaf extracts of Wedelia chinensis (Osbeck) Merrill. J Pharmaco Phytochem. 2014;2(6):29–33. [Google Scholar]
- 35.Laemmli UK. SDS–PAGE Laemmli method. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 36.Gutiérrez J, Avila C, Rojas E, Cerdas L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon. 1988;26(4):411–413. doi: 10.1016/0041-0101(88)90010-4. [DOI] [PubMed] [Google Scholar]
- 37.Vijayaraghavan P, Vincent SG. A simple method for the detection of protease activity on agar plates using Bromocresolgreen dye. J Biochem Technol. 2013;4(3):628–630. [Google Scholar]
- 38.Theakston RD, Reid HA. Development of simple standard assay procedures for the characterization of snake venoms. Bulletin of the world health organization. 1983;61(6):949–956. 6609011 [PMC free article] [PubMed] [Google Scholar]
- 39.Gené J, Roy A, Rojas G, Gutiérrez J, Cerdas L. Comparative study on coagulant, defibrinating, fibrinolytic and fibrinogenolytic activities of Costa Rican crotaline snake venoms and their neutralization by a polyvalent antivenom. Toxicon. 27(8):841–848. doi: 10.1016/0041-0101(89)90096-2. 19891; [DOI] [PubMed] [Google Scholar]
- 40.Lowry ОH, Rosebroug h NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;194(1):265–275. 14907713 [PubMed] [Google Scholar]
- 41.Liaqat A, Mallhi TH, Khan YH, Khokhar A, Chaman S, Ali M. Anti-snake venom properties of medicinal plants: A comprehensive systematic review of literature. Braz J Pharm Sci. 2022;58 doi: 10.1590/s2175-97902022e191124. [DOI] [Google Scholar]
- 42.Gnanaselvan S, Sivaraman T. Effect of aqueous root extract of Cynodon dactylon on the hemolytic activity of cardiotoxins from Indian cobra (Naja naja). J Appl Pharmaceut Sci. 2020;10(3):113–118. doi: 10.7324/JAPS.2020.103015. [DOI] [Google Scholar]