Abstract
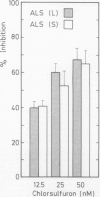
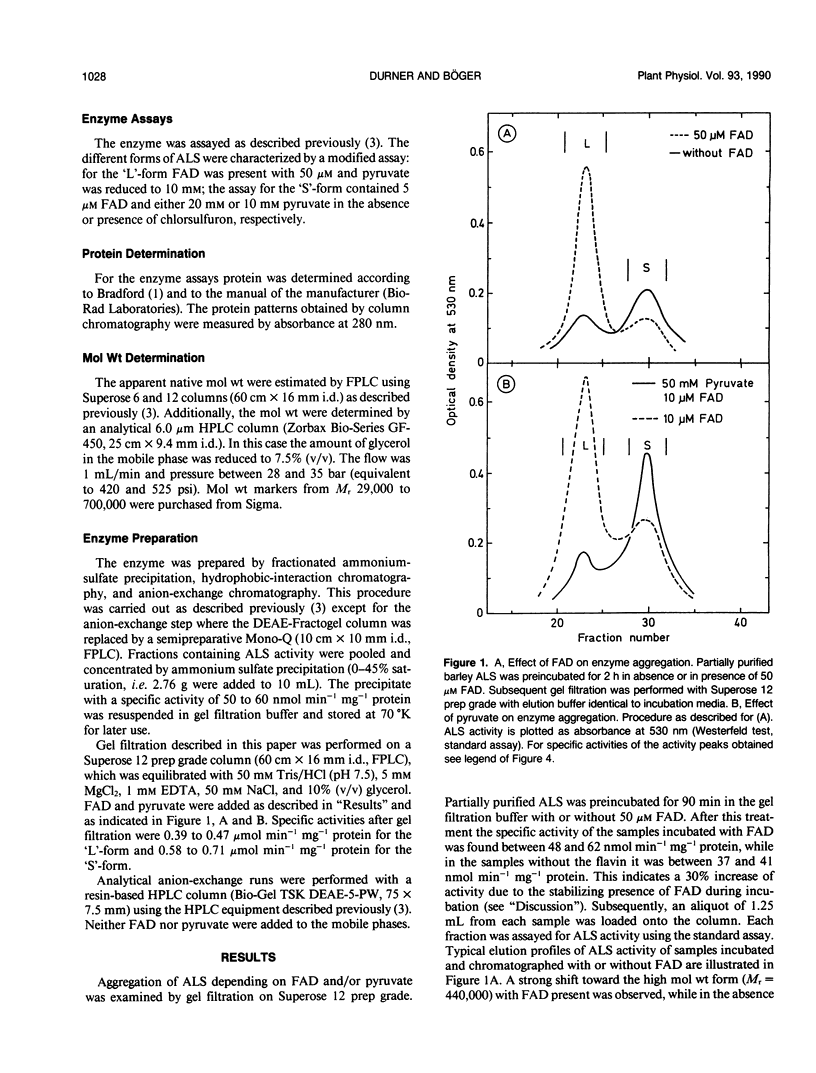
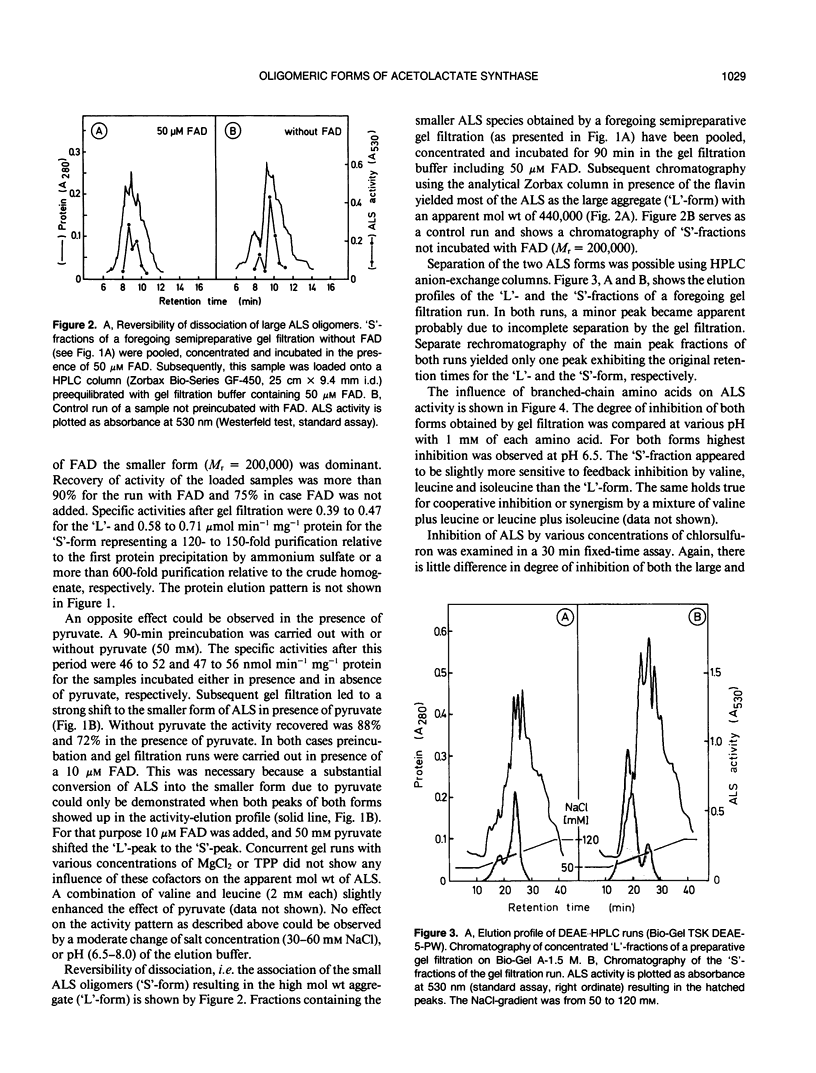
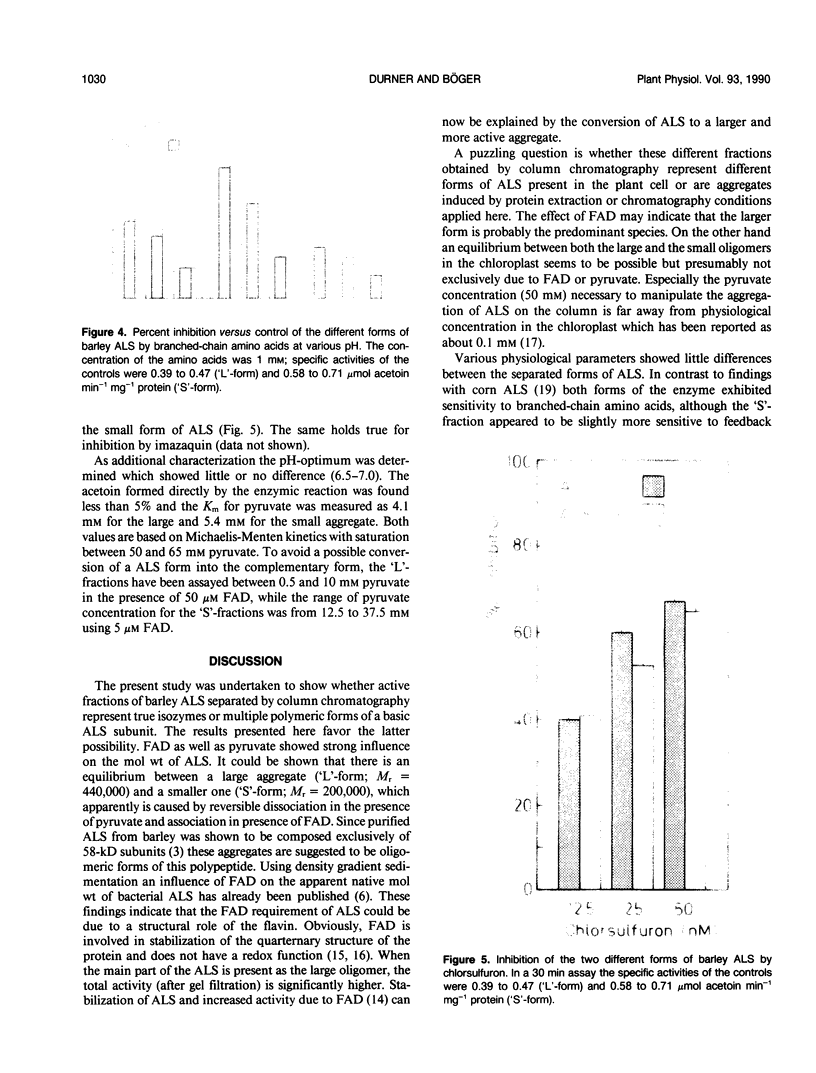
Acetolactate synthase (ALS, EC 4.1.3.18) has been extracted and partially purified from etiolated barley shoots (Hordeum vulgare L.). Multiple forms of this enzyme were separated by gel filtration and/or anion-exchange chromatography using fast protein liquid chromatography. It could be demonstrated that these two species are in equilibrium, which strongly depends on the structural role of flavin adenine dinucleotide and pyruvate. With 50 micromolar of flavin adenine dinucleotide in the medium most of the ALS aggregates as a high molecular weight form (Mr = 440,000), while 50 millimolar pyruvate facilitates dissociation into the smaller form (Mr = 200,000). Data are presented to show that two enzymatically active forms are not isozymes but different oligomeric species or aggregates of the basic 58-kilodalton subunit of ALS. These different ALS species exhibit little difference in feedback inhibition by valine, leucine and isoleucine or in inhibition by the sulfonylurea herbicide chlorsulfuron. Both aggregation forms show a broad pH-optimum between 6.5 and 7. Furthermore, the affinity for pyruvate and the amount of directly-formed acetoin indicate similar properties of these separated ALS forms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Eoyang L., Silverman P. M. Purification and subunit composition of acetohydroxyacid synthase I from Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):184–189. doi: 10.1128/jb.157.1.184-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimminger H., Umbarger H. E. Acetohydroxy acid synthase I of Escherichia coli: purification and properties. J Bacteriol. 1979 Feb;137(2):846–853. doi: 10.1128/jb.137.2.846-853.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F., Smith J. K. Isolation and characterization of plant genes coding for acetolactate synthase, the target enzyme for two classes of herbicides. Plant Physiol. 1987 Dec;85(4):1110–1117. doi: 10.1104/pp.85.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J. Cooperative feedback control of barley acetohydroxyacid synthetase by leucine, isoleucine, and valine. Arch Biochem Biophys. 1971 Oct;146(2):542–550. doi: 10.1016/0003-9861(71)90159-7. [DOI] [PubMed] [Google Scholar]
- Muhitch M. J. Acetolactate Synthase Activity in Developing Maize (Zea mays L.) Kernels. Plant Physiol. 1988 Jan;86(1):23–27. doi: 10.1104/pp.86.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan E. D., Kohlhaw G. B. Subcellular localization of isoleucine-valine biosynthetic enzymes in yeast. J Bacteriol. 1974 Nov;120(2):631–637. doi: 10.1128/jb.120.2.631-637.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss J. V., Van Dyk D. E., Vasta J. F., Kutny R. M. Purification and properties of Salmonella typhimurium acetolactate synthase isozyme II from Escherichia coli HB101/pDU9. Biochemistry. 1985 Aug 27;24(18):4952–4959. doi: 10.1021/bi00339a034. [DOI] [PubMed] [Google Scholar]
- Schulze-Siebert D., Heineke D., Scharf H., Schultz G. Pyruvate-Derived Amino Acids in Spinach Chloroplasts : Synthesis and Regulation during Photosynthetic Carbon Metabolism. Plant Physiol. 1984 Oct;76(2):465–471. doi: 10.1104/pp.76.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner D. L., Anderson P. C., Stidham M. A. Imidazolinones: potent inhibitors of acetohydroxyacid synthase. Plant Physiol. 1984 Oct;76(2):545–546. doi: 10.1104/pp.76.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]