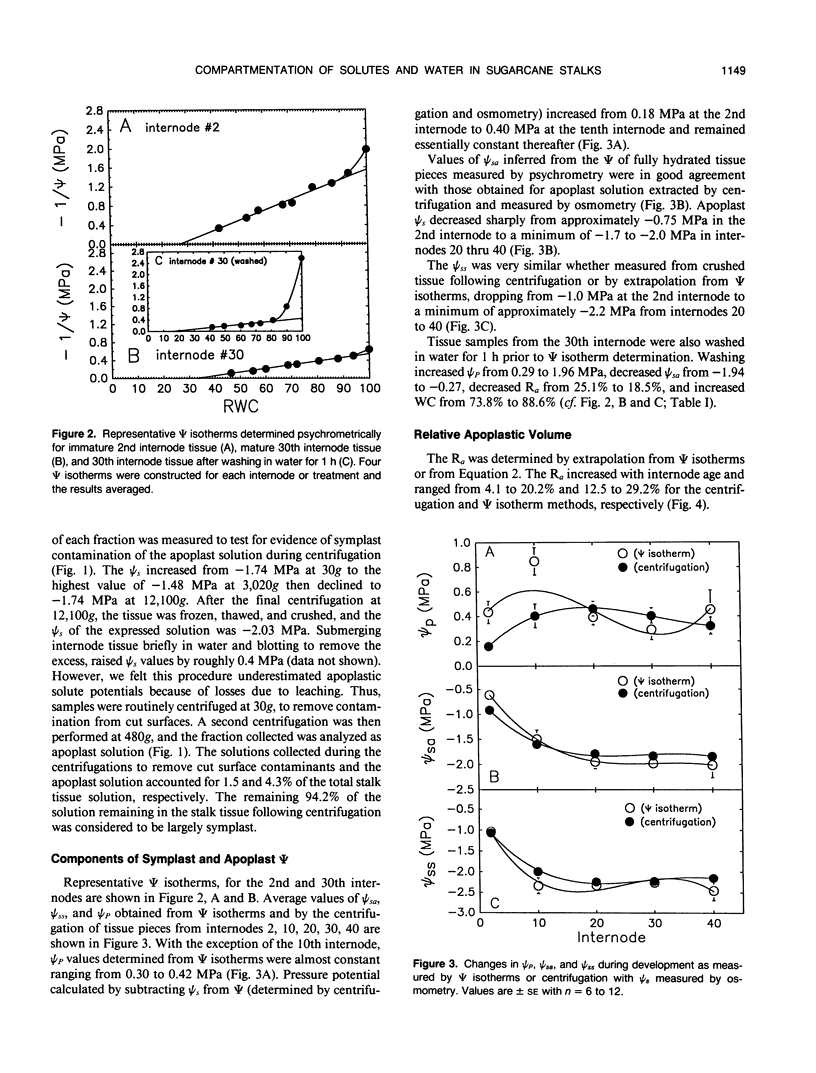
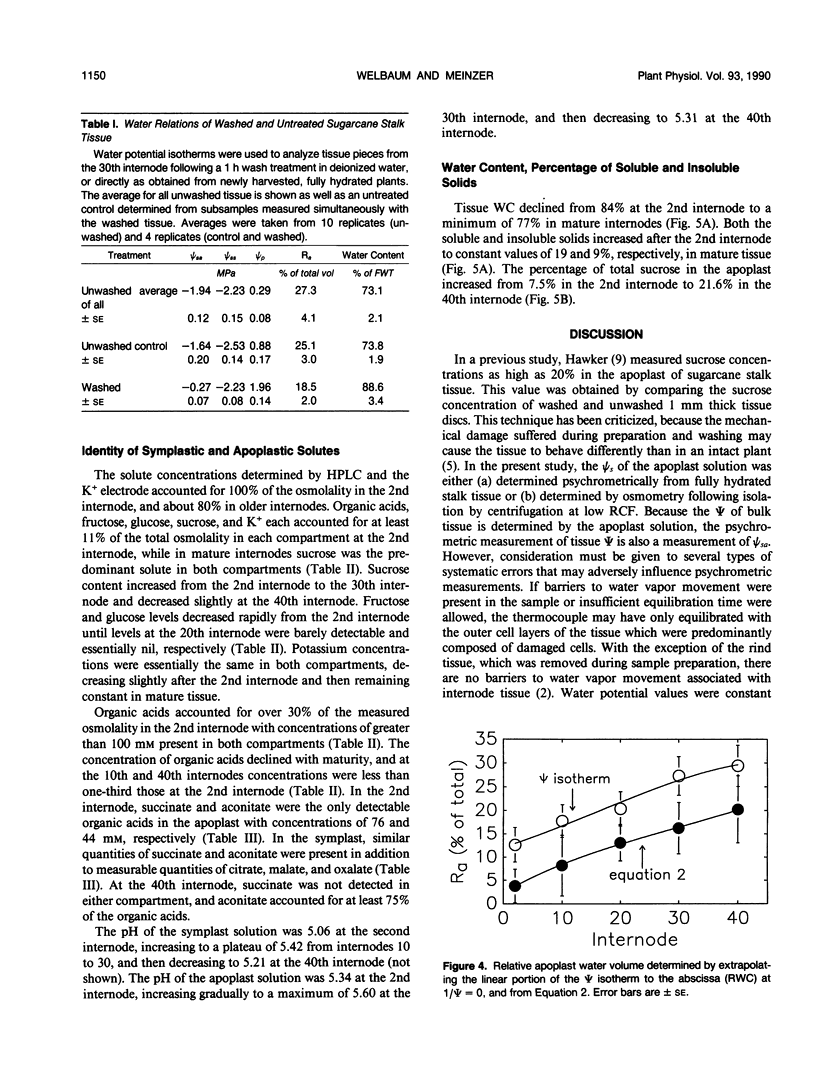
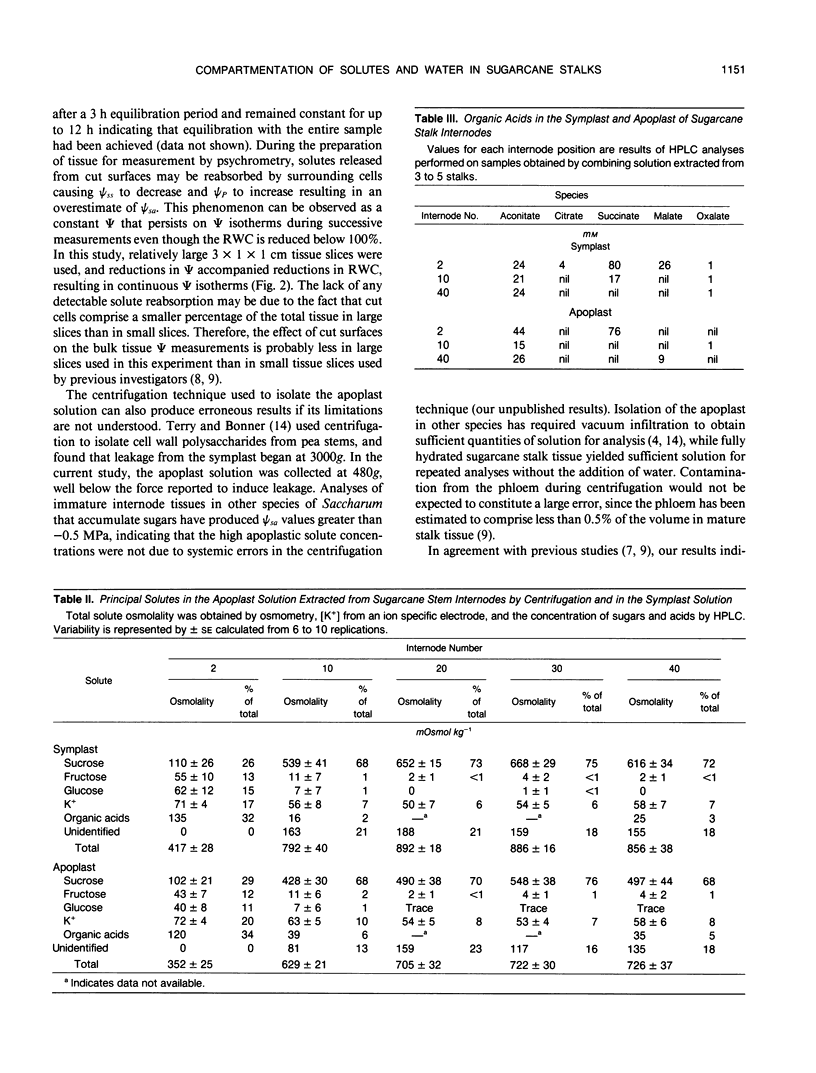
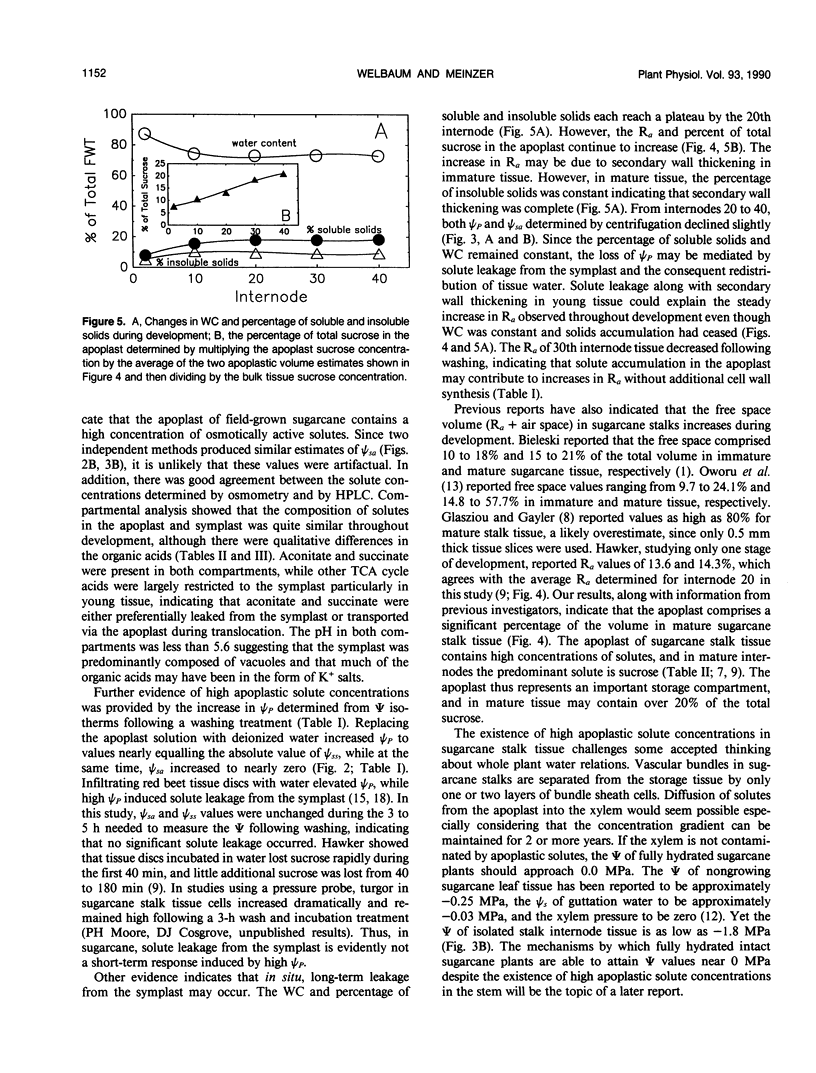
Abstract
Previous studies have suggested that the apoplast solution of sugarcane stalk tissue contains high concentrations of sucrose, but the accuracy of these reports has been questioned because sucrose leakage from damaged cells may have influenced the results. In this study, the solute potential of the apoplast and symplast of the second (immature), tenth, twentieth, thirtieth, and fortieth internodes of field-grown sugarcane (Saccharum spp. hybrid) stalk tissue was determined by two independent methods. Solute potential of the apoplast was measured either directly by osmometry from solution collected by centrifugation, or inferred from the initial water potential of fully hydrated tissue determined by thermocouple psychrometry before the tissue was progressively dehydrated for generation of water potential isotherms. Both methods produced nearly identical values ranging from −0.6 to −1.8 megapascals for immature and mature tissue, respectively. The solute potential of the symplast determined by either method ranged from −1.0 to approximately −2.2 megapascals for immature and mature internodes, respectively. Solute quantitation by HPLC agreed with concentrations inferred from osmometry. Washing thirtieth internode tissue in deionized water increased pressure potential from 0.29 to 1.96 megapascals. The apoplast of mature sugarcane stalk tissue is a significant storage compartment for sucrose containing as much as 25% of the total tissue water volume and as much as 21% of the stored sucrose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bull T. A., Gayler K. R., Glasziou K. T. Lateral movement of water and sugar across xylem in sugarcane stalks. Plant Physiol. 1972 Jun;49(6):1007–1011. doi: 10.1104/pp.49.6.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J., Cleland R. E. Solutes in the free space of growing stem tissues. Plant Physiol. 1983 Jun;72(2):326–331. doi: 10.1104/pp.72.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasziou K. T., Gayler K. R. Sugar accumulation in sugarcane: role of cell walls in sucrose transport. Plant Physiol. 1972 Jun;49(6):912–913. doi: 10.1104/pp.49.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer F. C., Moore P. H. Effect of apoplastic solutes on water potential in elongating sugarcane leaves. Plant Physiol. 1988 Mar;86(3):873–879. doi: 10.1104/pp.86.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry M. E., Bonner B. A. An Examination of Centrifugation as a Method of Extracting an Extracellular Solution from Peas, and Its Use for the Study of Indoleacetic Acid-induced Growth. Plant Physiol. 1980 Aug;66(2):321–325. doi: 10.1104/pp.66.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse R. E., Zamski E., Tomos A. D. Turgor regulation of sucrose transport in sugar beet taproot tissue. Plant Physiol. 1986 Jun;81(2):478–481. doi: 10.1104/pp.81.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]


